Clotrimazole and Betamethasone Dipropionate
These highlights do not include all the information needed to use CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE CREAM safely and effectively. See full prescribing information for CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE CREAM.CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE Cream, USP, for topical useInitial U.S. Approval: 1984
c6e4bf5f-f6d6-489e-9286-c30568c601b7
HUMAN PRESCRIPTION DRUG LABEL
Jan 1, 2021
Proficient Rx LP
DUNS: 079196022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Clotrimazole and Betamethasone Dipropionate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
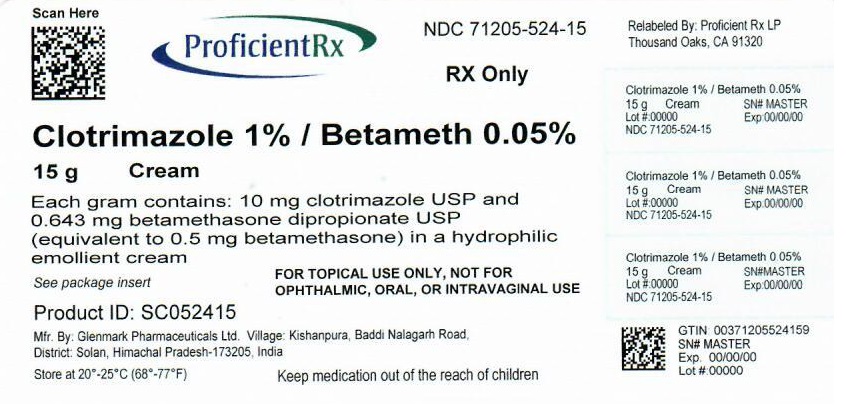
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 71205-524-15
Clotrimazole and Betamethasone Dipropionate Cream USP, 1%/0.05% (base)
FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. NOT RECOMMENDED FOR PATIENTS UNDER THE AGE OF 17 YEARS AND NOT RECOMMENDED FOR DIAPER DERMATITIS.
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Clotrimazole and betamethasone dipropionate cream is a combination of an azole antifungal and corticosteroid and is indicated for the topical treatment of symptomatic inflammatory tinea pedis, tinea cruris, and tinea corporis due to Epidermophyton floccosum, Trichophyton mentagrophytes, and Trichophyton rubrum in patients 17 years and older.
Clotrimazole and betamethasone dipropionate cream contains a combination of clotrimazole, an azole antifungal, and betamethasone dipropionate, a corticosteroid, and is indicated for the topical treatment of symptomatic inflammatory tinea pedis, tinea cruris, and tinea corporis due to Epidermophyton floccosum, Trichophyton mentagrophytes, and Trichophyton rubrum in patients 17 years and older. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
None. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Endocrine System
Clotrimazole and betamethasone dipropionate cream can cause reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency. This may occur during treatment or after withdrawal of treatment. Cushing’s syndrome and hyperglycemia may also occur due to the systemic effect of corticosteroids while on treatment. Factors that predispose a patient to HPA axis suppression include the use of high-potency steroids, large treatment surface areas, prolonged use, use of occlusive dressing, altered skin barrier, liver failure, and young age.
Because of the potential for systemic corticosteroid effects, patients may need to be periodically evaluated for HPA axis suppression. This may be done by using the adrenocorticotropic hormone (ACTH) stimulation test.
In a small trial, clotrimazole and betamethasone dipropionate cream was applied using large dosages, 7 g daily for 14 days (BID) to the crural area of normal adult subjects. Three of the 8 normal subjects on whom clotrimazole and betamethasone dipropionate cream was applied exhibited low morning plasma cortisol levels during treatment. One of these subjects had an abnormal cosyntropin test. The effect on morning plasma cortisol was transient and subjects recovered 1 week after discontinuing dosing. In addition, 2 separate trials in pediatric subjects demonstrated adrenal suppression as determined by cosyntropin testing [see Use in Specific Populations (8.4)].
If HPA axis suppression is documented, gradually withdraw the drug, reduce the frequency of application, or substitute with a less potent corticosteroid.
Pediatric patients may be more susceptible to systemic toxicity due to their larger skin-surface-to-body mass ratios [see Use in Specific Populations (8.4)].
5.2 Diaper Dermatitis
The use of clotrimazole and betamethasone dipropionate cream in the treatment of diaper dermatitis is not recommended.
5.3 Ophthalmic Adverse Reactions
Use of topical corticosteroids may increase the risk of posterior subcapsular cataracts and glaucoma. Cataracts and glaucoma have been reported in postmarketing experience with the use of topical corticosteroid products, including topical betamethasone products [see Adverse Reactions (6.2)].
Avoid contact of clotrimazole and betamethasone dipropionate cream with eyes. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
•
Clotrimazole and betamethasone dipropionate cream can cause reversible HPA axis suppression with the potential for glucocorticosteroid insufficiency during and after withdrawal of the treatment. Risk factor(s) are: use of high-potency topical corticosteroid, use over a large surface area or to areas under occlusion, prolonged use, altered skin barrier, liver failure, and young age. Modify use should HPA axis suppression develop. (5.1, 8.4)
•
Pediatric patients may be more susceptible to systemic toxicity. (5.1, 8.4)
•
The use of clotrimazole and betamethasone dipropionate cream in the treatment of diaper dermatitis is not recommended. (5.2)
•
Topical corticosteroid products may increase the risk of cataracts and glaucoma. If visual symptoms occur, consider referral to an ophthalmologist. (5.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials common adverse reaction reported for clotrimazole and betamethasone dipropionate cream was paresthesia in 1.9% of patients. Adverse reactions reported at a frequency < 1% included rash, edema, and secondary infection.
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following local adverse reactions have been reported with topical corticosteroids: itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, skin atrophy, striae, miliaria, capillary fragility (ecchymoses), telangiectasia, and sensitization (local reactions upon repeated application of product).
Ophthalmic adverse reactions of blurred vision, cataracts, glaucoma, increased intraocular pressure, and central serous chorioretinopathy have been reported with the use of topical corticosteroids, including topical betamethasone products.
Adverse reactions reported with the use of clotrimazole are: erythema, stinging, blistering, peeling, edema, pruritus, urticaria, and general irritation of the skin.
Most common adverse reactions reported for clotrimazole and betamethasone dipropionate cream were paraesthesia in 1.9% of patients and rash, edema, and secondary infections each in less than 1% of patients. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Glenmark Pharmaceuticals Inc., USA at 1 (888) 721-7115 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Treatment of tinea corporis or tinea cruris:
•
Apply a thin film of clotrimazole and betamethasone dipropionate cream into the affected skin areas twice a day for one week.
•
Do not use more than 45 grams per week. Do not use with occlusive dressings.
•
If a patient shows no clinical improvement after 1 week of treatment with clotrimazole and betamethasone dipropionate cream, the diagnosis should be reviewed.
•
Do not use longer than 2 weeks.
Treatment of tinea pedis:
•
Gently massage a sufficient amount of clotrimazole and betamethasone dipropionate cream into the affected skin areas twice a day for two weeks.
•
Do not use more than 45 grams per week. Do not use with occlusive dressings.
•
If a patient shows no clinical improvement after 2 weeks of treatment with clotrimazole and betamethasone dipropionate cream, the diagnosis should be reviewed.
•
Do not use longer than 4 weeks.
Clotrimazole and betamethasone dipropionate cream is for topical use only. It is not for oral, ophthalmic, or intravaginal use.
Avoid contact with eyes. Wash hands after each application.
•
Tinea pedis: Apply a thin film to the affected skin areas twice a day for 2 weeks. Do not use longer than 4 weeks. (2)
•
Tinea cruris and tinea corporis: Apply a thin film to the affected skin area twice a day for 1 week. Do not use longer than 2 weeks. (2)
•
Do not use with occlusive dressings unless directed by a physician. (2)
•
Not for ophthalmic, oral or intravaginal use. (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Cream, 1%/0.05%. Each gram of Clotrimazole and Betamethasone Dipropionate Cream USP, 1%/0.05% (base) contains 10 mg of clotrimazole, USP and 0.643 mg of betamethasone dipropionate, USP (equivalent to 0.5 mg of betamethasone) in a white to off-white, uniform and smooth cream base.
Cream, 1%/0.05%. (3)
Each gram of Clotrimazole and Betamethasone Dipropionate Cream USP, 1%/0.05% (base) contains 10 mg of clotrimazole, USP and 0.643 mg of betamethasone dipropionate, USP (equivalent to 0.5 mg of betamethasone). (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on topical betamethasone dipropionate or clotrimazole use in pregnant women to identify clotrimazole and betamethasone dipropionate cream associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Observational studies suggest an increased risk of low birthweight infants with the use of potent or very potent topical corticosteroid during pregnancy. Advise pregnant women that clotrimazole and betamethasone dipropionate cream may increase the risk of having a low birthweight infant and to use clotrimazole and betamethasone dipropionate cream on the smallest area of skin and for the shortest duration possible.
There have been no reproduction studies performed in animals or humans with the combination of clotrimazole and betamethasone dipropionate. In an animal reproduction study, betamethasone dipropionate caused malformations (i.e., umbilical hernias, cephalocele, and cleft palate) in pregnant rabbits when given by the intramuscular route during organogenesis [see Data]. The available data do not allow the calculation of relevant comparisons between the systemic exposure of clotrimazole and/or betamethasone dipropionate observed in the animal studies to the systemic exposure that would be expected in humans after topical use of clotrimazole and betamethasone dipropionate cream.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Clotrimazole
Studies in pregnant rats treated during organogenesis with intravaginal doses up to 100 mg/kg/day revealed no evidence of fetotoxicity due to clotrimazole exposure.
No increase in fetal malformations was noted in pregnant rats receiving oral (gastric tube) clotrimazole doses up to 100 mg/kg/day during gestation Days 6 to 15. However, clotrimazole dosed at 100 mg/kg/day was embryotoxic (increased resorptions), fetotoxic (reduced fetal weights), and maternally toxic (reduced body weight gain) to rats. Clotrimazole dosed at 200 mg/kg/day was maternally lethal, and therefore, fetuses were not evaluated in this group. Also in this study, doses up to 50 mg/kg/day had no adverse effects on dams or fetuses. However, in the combined fertility, embryofetal development, and postnatal development study conducted in rats, 50 mg/kg/day clotrimazole was associated with reduced maternal weight gain and reduced numbers of offspring reared to 4 weeks [see Nonclinical Toxicology (13.1)].
Oral clotrimazole doses of 25, 50, 100, and 200 mg/kg/day did not cause malformations in pregnant mice. No evidence of maternal toxicity or embryotoxicity was seen in pregnant rabbits dosed orally during organogenesis with 60, 120, or 180 mg/kg/day.
Betamethasone Dipropionate
Betamethasone dipropionate caused malformations when given to pregnant rabbits during organogenesis by the intramuscular route at doses of 0.05 mg/kg/day. The abnormalities observed included umbilical hernias, cephalocele, and cleft palates.
8.2 Lactation
Risk Summary
There are no data regarding the excretion of betamethasone dipropionate or clotrimazole into breast milk, the effects on the breastfed infant, or the effects on milk production after topical application to women who are breastfeeding.
It is possible that topical administration of betamethasone dipropionate could result in sufficient systemic absorption to produce detectable quantities in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for clotrimazole and betamethasone dipropionate cream and any potential adverse effects on the breastfed infant from clotrimazole and betamethasone dipropionate cream or from the underlying maternal condition.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breast milk, use clotrimazole and betamethasone dipropionate cream on the smallest area of skin and for the shortest duration possible while breastfeeding. Advise breastfeeding women not to apply clotrimazole and betamethasone dipropionate cream directly to the nipple and areola to avoid direct infant exposure [see Use in Specific Populations (8.4)].
8.4 Pediatric Use
The use of clotrimazole and betamethasone dipropionate cream in patients under 17 years of age is not recommended.
Adverse events consistent with corticosteroid use have been observed in pediatric patients treated with clotrimazole and betamethasone dipropionate cream. In open-label trials, 17 of 43 (39.5%) evaluable pediatric subjects (aged 12 to 16 years old) using clotrimazole and betamethasone dipropionate cream for treatment of tinea pedis demonstrated adrenal suppression as determined by cosyntropin testing. In another open-label trial, 8 of 17 (47.1%) evaluable pediatric subjects (aged 12 to 16 years old) using clotrimazole and betamethasone dipropionate cream for treatment of tinea cruris demonstrated adrenal suppression as determined by cosyntropin testing.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression when they are treated with topical corticosteroids. They are, therefore also at greater risk of adrenal insufficiency during and/or after withdrawal of treatment. Pediatric patients may be more susceptible than adults to skin atrophy, including striae, when they are treated with topical corticosteroids.
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids [see Warnings and Precautions (5.1)].
Avoid use of clotrimazole and betamethasone dipropionate cream in the treatment of diaper dermatitis.
8.5 Geriatric Use
Clinical studies of clotrimazole and betamethasone dipropionate cream did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, greater sensitivity of some older individuals cannot be ruled out. The use of clotrimazole and betamethasone dipropionate cream under occlusion, such as in diaper dermatitis, is not recommended.
Postmarket adverse event reporting for clotrimazole and betamethasone dipropionate cream in patients aged 65 and above includes reports of skin atrophy and rare reports of skin ulceration. Caution should be exercised with the use of these corticosteroid-containing topical products on thinning skin.
DESCRIPTION SECTION
11 DESCRIPTION
Clotrimazole and Betamethasone Dipropionate Cream USP, 1%/0.05% (base), contains combinations of clotrimazole, USP, an azole antifungal, and betamethasone dipropionate, USP, a corticosteroid, for topical use.
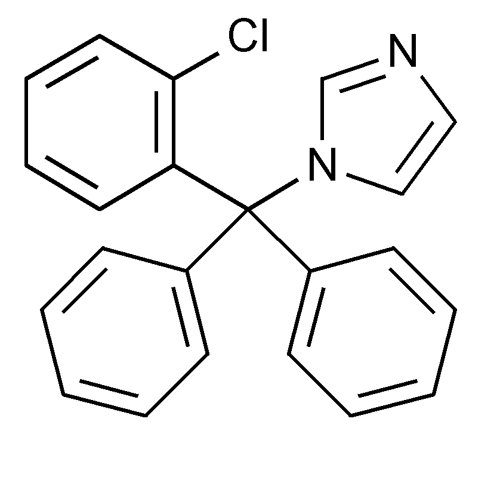
Chemically, clotrimazole, USP is 1-[(2-Chlorophenyl)diphenylmethyl]-1H-imidazole, with the empirical formula C22H17ClN2, a molecular weight of 344.84 g/mol, and the following structural formula:
Clotrimazole, USP is a white to pale yellow, crystalline powder, odorless, practically insoluble in water, freely soluble in methanol, in acetone, in chloroform and in alcohol.
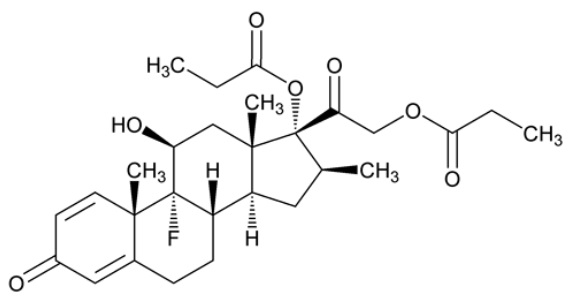
Betamethasone dipropionate, USP has the chemical name 9-fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate, with the empirical formula C28H37FO7, a molecular weight of 504.60 g/mol, and the following structural formula:
Betamethasone dipropionate, USP is a white to almost white crystalline powder, practically insoluble in water, freely soluble in acetone and in methylene chloride, sparingly soluble in alcohol.
Each gram of Clotrimazole and Betamethasone Dipropionate Cream USP, 1%/0.05% (base) contains 10 mg clotrimazole, USP and 0.643 mg betamethasone dipropionate, USP (equivalent to 0.5 mg betamethasone), in a white to off- white, uniform and smooth cream consisting of benzyl alcohol, ceteareth-30, cetyl alcohol, mineral oil, monobasic sodium phosphate, phosphoric acid, propylene glycol, purified water, sodium hydroxide, stearyl alcohol, and white petrolatum.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clotrimazole is an azole antifungal [see Clinical Pharmacology (12.4)].
Betamethasone dipropionate is a corticosteroid. Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action for the treatment of tinea pedis, tinea cruris and tinea corporis is unknown.
12.2 Pharmacodynamics
Vasoconstrictor Assay:
Studies performed with clotrimazole and betamethasone dipropionate cream indicate that these topical combination antifungal/corticosteroids may have vasoconstrictor potencies in a range that is comparable to high-potency topical corticosteroids. However, similar blanching scores do not necessarily imply therapeutic equivalence.
12.3 Pharmacokinetics
Skin penetration and systemic absorption of clotrimazole and betamethasone dipropionate following topical application of clotrimazole and betamethasone dipropionate cream has not been studied.
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption of topical corticosteroids. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids [see Dosage and Administration (2)].
Once absorbed through the skin, the pharmacokinetics of topical corticosteroids are similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
12.4 Microbiology
Mechanism of Action:
Clotrimazole, an azole antifungal agent, inhibits 14-α-demethylation of lanosterol in fungi by binding to one of the cytochrome P-450 enzymes. This leads to the accumulation of 14-α-methylsterols and reduced concentrations of ergosterol, a sterol essential for a normal fungal cytoplasmic membrane. The methylsterols may affect the electron transport system, thereby inhibiting growth of fungi.
Activity In Vitro and In Vivo:
Clotrimazole has been shown to be active against most strains of the following dermatophytes, both in vitro and in clinical infections, Epidermophyton floccosum, Trichophyton mentagrophytes, and Trichophyton rubrum [see Indications and Usage (1)].
Drug Resistance:
Strains of dermatophytes having a natural resistance to clotrimazole have not been reported. Resistance to azoles, including clotrimazole, has been reported in some Candida species.
No single-step or multiple-step resistance to clotrimazole has developed during successive passages of Trichophyton mentagrophytes.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of the combination of clotrimazole and betamethasone dipropionate or either component individually.
Betamethasone was negative in the bacterial mutagenicity assay (Salmonella typhimurium and Escherichia coli) and in the mammalian cell mutagenicity assay (CHO/HGPRT). It was positive in the in vitro human lymphocyte chromosome aberration assay, and equivocal in the in vivo mouse bone marrow micronucleus assay.
In a combined study of the effects of clotrimazole on fertility, embryofetal development, and postnatal development, male and female rats were dosed orally (diet admixture) with dose levels of 5, 10, 25, or 50 mg/kg/day from 10 weeks prior to mating until 4 weeks postpartum. No adverse effects on the duration of estrous cycle, fertility, or duration of pregnancy were noted.
Reproductive studies with betamethasone dipropionate conducted in rabbits at doses of 1 mg/kg/day by the intramuscular route and in mice up to 33 mg/kg/day by the intramuscular route indicated no impairment of fertility except for dose-related increases in fetal resorption rates in both species.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
In clinical trials of tinea corporis, tinea cruris, and tinea pedis, subjects treated with clotrimazole and betamethasone dipropionate cream showed a better clinical response at the first return visit than subjects treated with clotrimazole cream. In tinea corporis and tinea cruris, the subject returned 3 to 5 days after starting treatment, and in tinea pedis, after 1 week. Mycological cure rates observed in subjects treated with clotrimazole and betamethasone dipropionate cream were as good as, or better than, in those subjects treated with clotrimazole cream. In these same clinical studies, patients treated with clotrimazole and betamethasone dipropionate cream showed better clinical responses and mycological cure rates when compared with subjects treated with betamethasone dipropionate cream.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Clotrimazole and Betamethasone Dipropionate Cream USP, 1%/0.05% is white to off-white, uniform and smooth cream and supplied in
NDC 71205-524-15 15 gram tube (1 tube per carton)
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not keep in cold or hot weather conditions.
Rx only
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform the patient of the following:
Pregnancy
Advise pregnant women that clotrimazole and betamethasone dipropionate cream may increase the risk of having a low birthweight infant and to use clotrimazole and betamethasone dipropionate cream on the smallest area of skin and for the shortest duration possible [see Use in Specific Populations (8.1)].
Lactation
Advise a woman to use clotrimazole and betamethasone dipropionate cream on the smallest area of skin and for the shortest duration possible while breastfeeding. Advise breastfeeding women not to apply clotrimazole and betamethasone dipropionate cream directly to the nipple and areola to avoid direct infant exposure [see Use in Specific Populations (8.2)].
Important Administration Instructions
Inform patients of the following:
•
Use clotrimazole and betamethasone dipropionate cream as directed by the physician. It is for external use only.
•
Avoid contact with the eyes, the mouth, or intravaginally.
•
Advise patients to report any visual symptoms to their healthcare providers.
•
Do not use clotrimazole and betamethasone dipropionate cream on the face or underarms.
•
Do not use more than 45 grams of clotrimazole and betamethasone dipropionate cream per week.
•
When using clotrimazole and betamethasone dipropionate cream in the groin area, patients should use the medication for 2 weeks only, and apply the cream sparingly. Patients should wear loose-fitting clothing. Notify the physician if the condition persists after 2 weeks.
•
Do not use clotrimazole and betamethasone dipropionate cream for any disorder other than that for which it was prescribed.
•
Do not bandage, cover or wrap the treatment area unless directed by the physician. Avoid use of clotrimazole and betamethasone dipropionate cream in the diaper area, as diapers or plastic pants may constitute occlusive dressing.
•
Report any signs of local adverse reactions to the physician. Advise patients that local reactions and skin atrophy are more likely to occur with occlusive use or prolonged use.
•
This medication is to be used for the full prescribed treatment time, even though the symptoms may have improved. Notify the physician if there is no improvement after 1 week of treatment for tinea cruris or tinea corporis, or after 2 weeks for tinea pedis.
Manufactured by:
Glenmark Pharmaceuticals Limited
Baddi, Himachal Pradesh 173205, India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
Relabeled by:
Proficient Rx LP
Thousand Oaks, CA 91320
November 2020
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
Clotrimazole (kloe trim' a zole) and Betamethasone Dipropionate (bay" ta meth' a sone dye proe' pee oh nate)
Cream USP, 1%/0.05%
Important information: Clotrimazole and betamethasone dipropionate cream is for use on skin only. Do not use clotrimazole and betamethasone dipropionate cream in your eyes, mouth, or vagina.
What is clotrimazole and betamethasone dipropionate cream?
•
Clotrimazole and betamethasone dipropionate cream is a prescription medication used on the skin (topical) to treat fungal infections of the feet, groin, and body in people 17 years of age and older. Clotrimazole and betamethasone dipropionate cream is used for fungal infections that are inflamed and have symptoms of redness or itching.
•
Clotrimazole and betamethasone dipropionate cream should not be used in children under 17 years of age.
Before using clotrimazole and betamethasone dipropionate cream, tell your healthcare provider about all your medical conditions, including if you:
•
are pregnant or plan to become pregnant. It is not known if clotrimazole and betamethasone dipropionate cream will harm your unborn baby. If you use clotrimazole and betamethasone dipropionate cream during pregnancy, use clotrimazole and betamethasone dipropionate cream on the smallest area of the skin and for the shortest time needed.
•
are breastfeeding or plan to breastfeed. It is not known if clotrimazole and betamethasone dipropionate cream passes into your breast milk. Breastfeeding women should use clotrimazole and betamethasone dipropionate cream on the smallest area of skin and for the shortest time needed while breastfeeding. Do not apply clotrimazole and betamethasone dipropionate cream directly to the nipple and areola to avoid contact with your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take other corticosteroid medicines by mouth or use other products on your skin or scalp that contain corticosteroids.
How should I use clotrimazole and betamethasone dipropionate cream?
•
Use clotrimazole and betamethasone dipropionate cream exactly as your healthcare provider tells you to use it.
•
Use clotrimazole and betamethasone dipropionate cream for the prescribed treatment time, even if your symptoms get better.
•
Do not use more than 45 grams of clotrimazole and betamethasone dipropionate cream in 1 week.
•
Do not bandage, cover, or wrap the treated area unless your healthcare provider tells you to. Wear loose-fitting clothing if you use clotrimazole and betamethasone dipropionate cream in the groin area.
•
Do not use clotrimazole and betamethasone dipropionate cream on your face or underarms (armpits).
•
**For treatment of fungal infections of the groin and body:**
•
Apply a thin layer of clotrimazole and betamethasone dipropionate cream to the affected skin area 2 times a day for 1 week.
•
Tell your healthcare provider if the treated skin area does not improve after 1 week of treatment.
•
Do not use clotrimazole and betamethasone dipropionate cream for longer than 2 weeks.
•
**For treatment of fungal infections of the feet:**
•
Apply a thin layer of clotrimazole and betamethasone dipropionate cream to the affected skin area 2 times a day for 2 weeks.
•
Tell your healthcare provider if the treated skin area does not improve after 2 weeks of treatment. Do not use clotrimazole and betamethasone dipropionate cream longer than 4 weeks.
•
Wash your hands after applying clotrimazole and betamethasone dipropionate cream.
What should I avoid while using clotrimazole and betamethasone dipropionate cream?
Clotrimazole and betamethasone dipropionate cream should not be used to treat diaper rash or redness. You should avoid applying clotrimazole and betamethasone dipropionate cream in the diaper area.
What are the possible side effects of clotrimazole and betamethasone dipropionate cream?
Clotrimazole and betamethasone dipropionate cream may cause serious side effects, including:
•
C**lotrimazole and betamethasone dipropionate cream can pass through your skin**. Too much clotrimazole and betamethasone dipropionate cream passing through your skin can cause your adrenal glands to stop working. Your healthcare provider may do blood tests to check for adrenal gland problems.
•
**Vision problems**. Topical corticosteroids may increase your chance of developing cataracts and glaucoma. Tell your healthcare provider if you develop blurred vision or other vision problems during treatment with clotrimazole and betamethasone dipropionate cream.
The most common side effects of clotrimazole and betamethasone dipropionate cream include burning, tingling, rash, swelling, and infections. These are not all the possible side effects of clotrimazole and betamethasone dipropionate cream.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store clotrimazole and betamethasone dipropionate cream?
•
Store clotrimazole and betamethasone dipropionate cream at room temperature between 68°F to 77°F (20°C to 25°C).
•
Do not leave clotrimazole and betamethasone dipropionate cream in your car in cold or hot weather conditions.
•
Keep clotrimazole and betamethasone dipropionate cream and all medicines out of the reach of children.
General information about the safe and effective use of clotrimazole and betamethasone dipropionate cream.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use clotrimazole and betamethasone dipropionate cream for a condition for which it was not prescribed. Do not give clotrimazole and betamethasone dipropionate cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about clotrimazole and betamethasone dipropionate cream that is written for health professionals.
What are the ingredients in clotrimazole and betamethasone dipropionate cream?
Active ingredients: clotrimazole and betamethasone dipropionate
Inactive ingredients: benzyl alcohol, ceteareth-30, cetyl alcohol, mineral oil, monobasic sodium phosphate, phosphoric acid, propylene glycol, purified water, sodium hydroxide, stearyl alcohol, and white petrolatum.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Glenmark Pharmaceuticals Limited
Baddi, Himachal Pradesh 173205, India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
Relabeled by:
Proficient Rx LP
Thousand Oaks, CA 91320
November 2020
