Piperacillin and Tazobactam
These highlights do not include all the information needed to use PIPERACILLIN and TAZOBACTAM FOR INJECTION safely and effectively. See full prescribing information for PIPERACILLIN and TAZOBACTAM FOR INJECTION. PIPERACILLIN and TAZOBACTAM, for Injection, for intravenous use, pharmacy bulk bottles. Initial U.S. Approval: 1993
68d5384d-1691-4afc-8a1a-488f8e2ef2c5
HUMAN PRESCRIPTION DRUG LABEL
Feb 26, 2025
Provepharm Inc.
DUNS: 086861066
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Piperacillin sodium and Tazobactam sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Piperacillin sodium and Tazobactam sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
OVERDOSAGE SECTION
10 OVERDOSAGE
There have been postmarketing reports of overdose with piperacillin/tazobactam. The majority of those events experienced, including nausea, vomiting, and diarrhea, have also been reported with the usual recommended dosages. Patients may experience neuromuscular excitability or seizures if higher than recommended doses are given intravenously (particularly in the presence of renal failure) [see Warnings and Precautions (5.5)].
Treatment should be supportive and symptomatic according the patient's clinical presentation. Excessive serum concentrations of either piperacillin or tazobactam may be reduced by hemodialysis. Following a single 3.375 g dose of piperacillin/tazobactam, the percentage of the piperacillin and tazobactam dose removed by hemodialysis was approximately 31% and 39%, respectively [see Clinical Pharmacology (12)].
DESCRIPTION SECTION
11 DESCRIPTION
Piperacillin and Tazobactam for Injection is an injectable antibacterial combination product consisting of the semisynthetic antibacterial piperacillin sodium and the beta-lactamase inhibitor tazobactam sodium for intravenous administration.
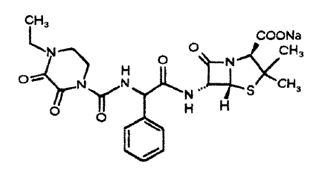
Piperacillin sodium is derived from D(-)-α-aminobenzyl-penicillin. The chemical name of piperacillin sodium is sodium(2S,5R,6R)-6-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate. The chemical formula is C23H26N5NaO7S and the molecular weight is 539.5. The chemical structure of piperacillin sodium is:
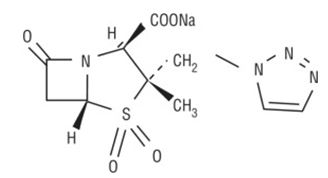
Tazobactam sodium, a derivative of the penicillin nucleus, is a penicillanic acid sulfone. Its chemical name is sodium(2S,3S,5R)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate-4,4-dioxide. The chemical formula is C10H11N4NaO5S and the molecular weight is 322.3. The chemical structure of tazobactam sodium is:
Piperacillin and Tazobactam for Injection contains a total of 2.84 mEq (65 mg) of sodium (Na+) per gram of piperacillin in the combination product.
Piperacillin and Tazobactam for Injection is a white to off-white sterile powder consisting of piperacillin and tazobactam as their sodium salts packaged in a glass bottles.
- Each Piperacillin and Tazobactam for Injection 13.5 g pharmacy bulk bottle contains piperacillin sodium equivalent to 12 grams of piperacillin and tazobactam sodium equivalent to 1.5 g of tazobactam sufficient for delivery of multiple doses.
- Each Piperacillin and Tazobactam for Injection 40.5 g pharmacy bulk bottle contains piperacillin sodium equivalent to 36 grams of piperacillin and tazobactam sodium equivalent to 4.5 g of tazobactam sufficient for delivery of multiple doses.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Piperacillin and tazobactam for injection is an antibacterial drug [see Microbiology (12.4)].
12.2 Pharmacodynamics
The pharmacodynamic parameter for piperacillin/tazobactam that is most predictive of clinical and microbiological efficacy is time above MIC.
12.3 Pharmacokinetics
The mean and coefficients of variation (CV%) for the pharmacokinetic parameters of piperacillin and tazobactam for injection after multiple intravenous doses are summarized in Table 7.
Table 7: Mean (CV%) Piperacillin and Tazobactam for Injection PK Parameters|
a Piperacillin and tazobactam were given in combination, infused over 30
minutes. | ||||||
|
** Piperacillin** | ||||||
|
Piperacillin/Tazobactam |
Cmax (mcg/mL) |
AUCb (mcg•h/mL) |
CL (mL/min) |
V (L) |
T1/2 (h) |
CLR (mL/min) |
|
2.25 g |
134 |
131 [14] |
257 |
17.4 |
0.79 |
|
|
3.375 g |
242 |
242 [10] |
207 |
15.1 |
0.84 |
140 |
|
4.5 g |
298 |
322 [16] |
210 |
15.4 |
0.84 |
|
|
Tazobactam | ||||||
|
Piperacillin/Tazobactam |
Cmax (mcg/mL) |
AUCb (mcg•h/mL) |
CL (mL/min) |
V (L) |
T1/2 (h) |
CLR (mL/min) |
|
2.25 g |
15 |
16.0 [21] |
258 |
17.0 |
0.77 |
|
|
3.375 g |
24 |
25.0 [8] |
251 |
14.8 |
0.68 |
166 |
|
4.5 g |
34 |
39.8 [15] |
206 |
14.7 |
0.82 |
|
Peak plasma concentrations of piperacillin and tazobactam are attained immediately after completion of an intravenous infusion of piperacillin and tazobactam for injection. Piperacillin plasma concentrations, following a 30-minute infusion of piperacillin and tazobactam for injection, were similar to those attained when equivalent doses of piperacillin were administered alone. Steady-state plasma concentrations of piperacillin and tazobactam were similar to those attained after the first dose due to the short half-lives of piperacillin and tazobactam.
Distribution
Both piperacillin and tazobactam are approximately 30% bound to plasma proteins. The protein binding of either piperacillin or tazobactam is unaffected by the presence of the other compound. Protein binding of the tazobactam metabolite is negligible.
Piperacillin and tazobactam are widely distributed into tissues and body fluids including intestinal mucosa, gallbladder, lung, female reproductive tissues (uterus, ovary, and fallopian tube), interstitial fluid, and bile. Mean tissue concentrations are generally 50% to 100% of those in plasma. Distribution of piperacillin and tazobactam into cerebrospinal fluid is low in subjects with non-inflamed meninges, as with other penicillins (see Table 8).
Table 8: Piperacillin/Tazobactam Concentrations in Selected Tissues and Fluids after Single 4 g/0.5 g 30-min IV Infusion of Piperacillin and Tazobactam for Injection|
a Each subject provided a single sample. | ||||||
|
Tissue or |
** N****a** |
** Sampling periodb**** |
** Mean PIP** |
** Tissue:** |
Tazo |
** Tazo Tissue:** |
|
Skin |
35 |
0.5 - 4.5 |
34.8 - 94.2 |
0.60 - 1.1 |
4.0 - 7.7 |
0.49 - 0.93 |
|
Fatty |
37 |
0.5 - 4.5 |
4.0 - 10.1 |
0.097 - 0.115 |
0.7 - 1.5 |
0.10 - 0.13 |
|
Muscle |
36 |
0.5 - 4.5 |
9.4 - 23.3 |
0.29 - 0.18 |
1.4 - 2.7 |
0.18 - 0.30 |
|
Proximal |
7 |
1.5 - 2.5 |
31.4 |
0.55 |
10.3 |
1.15 |
|
Distal |
7 |
1.5 - 2.5 |
31.2 |
0.59 |
14.5 |
2.1 |
|
Appendix |
22 |
0.5 - 2.5 |
26.5 - 64.1 |
0.43 - 0.53 |
9.1 - 18.6 |
0.80 - 1.35 |
Metabolism
Piperacillin is metabolized to a minor microbiologically active desethyl metabolite. Tazobactam is metabolized to a single metabolite that lacks pharmacological and antibacterial activities.
Excretion
Following single or multiple piperacillin and tazobactam for injection doses to healthy subjects, the plasma half-life of piperacillin and of tazobactam ranged from 0.7 to 1.2 hours and was unaffected by dose or duration of infusion.
Both piperacillin and tazobactam are eliminated via the kidney by glomerular filtration and tubular secretion. Piperacillin is excreted rapidly as unchanged drug with 68% of the administered dose excreted in the urine. Tazobactam and its metabolite are eliminated primarily by renal excretion with 80% of the administered dose excreted as unchanged drug and the remainder as the single metabolite. Piperacillin, tazobactam and desethyl piperacillin are also secreted into the bile.
Specific Populations
Renal Impairment
After the administration of single doses of piperacillin/tazobactam to subjects with renal impairment, the half-life of piperacillin and of tazobactam increases with decreasing creatinine clearance. At creatinine clearance below 20 mL/min, the increase in half-life is twofold for piperacillin and fourfold for tazobactam compared to subjects with normal renal function. Dosage adjustments for piperacillin and tazobactam for injection are recommended when creatinine clearance is below 40 mL/min in patients receiving the usual recommended daily dose of piperacillin and tazobactam for injection. See Dosage and Administration (2) for specific recommendations for the treatment of patients with renal-impairment.
Hemodialysis removes 30% to 40% of a piperacillin/tazobactam dose with an additional 5% of the tazobactam dose removed as the tazobactam metabolite. Peritoneal dialysis removes approximately 6% and 21% of the piperacillin and tazobactam doses, respectively, with up to 16% of the tazobactam dose removed as the tazobactam metabolite. For dosage recommendations for patients undergoing hemodialysis [see Dosage and Administration (2)].
Hepatic Impairment
The half-life of piperacillin and of tazobactam increases by approximately 25% and 18%, respectively, in patients with hepatic cirrhosis compared to healthy subjects. However, this difference does not warrant dosage adjustment of piperacillin and tazobactam for injection due to hepatic cirrhosis.
Pediatrics
Piperacillin and tazobactam pharmacokinetics were studied in pediatric patients 2 months of age and older. The clearance of both compounds is slower in the younger patients compared to older children and adults.
In a population PK analysis, estimated clearance for 9 month-old to 12 year- old patients was comparable to adults, with a population mean (SE) value of 5.64 (0.34) mL/min/kg. The piperacillin clearance estimate is 80% of this value for pediatric patients 2-9 months old. In patients younger than 2 months of age, clearance of piperacillin is slower compared to older children; however, it is not adequately characterized for dosing recommendations. The population mean (SE) for piperacillin volume of distribution is 0.243 (0.011) L/kg and is independent of age.
Geriatrics
The impact of age on the pharmacokinetics of piperacillin and tazobactam was evaluated in healthy male subjects, aged 18-35 years (n=6) and aged 65 to 80 years (n=12). Mean half-life for piperacillin and tazobactam was 32% and 55% higher, respectively, in the elderly compared to the younger subjects. This difference may be due to age-related changes in creatinine clearance.
Race
The effect of race on piperacillin and tazobactam was evaluated in healthy male volunteers. No difference in piperacillin or tazobactam pharmacokinetics was observed between Asian (n=9) and Caucasian (n=9) healthy volunteers who received single 4/0.5 g doses.
Drug Interactions
The potential for pharmacokinetic drug interactions between piperacillin and tazobactam for injection and aminoglycosides, probenecid, vancomycin, heparin, vecuronium, and methotrexate has been evaluated [see Drug Interactions (7)].
12.4 Microbiology
Mechanism of Action
Piperacillin sodium exerts bactericidal activity by inhibiting septum formation and cell wall synthesis of susceptible bacteria. In vitro, piperacillin is active against a variety of gram-positive and gram-negative aerobic and anaerobic bacteria. Tazobactam sodium has little clinically relevant in vitro activity against bacteria due to its reduced affinity to penicillin-binding proteins. It is, however, a beta-lactamase inhibitor of the Molecular Class A enzymes, including Richmond-Sykes Class III (Bush class 2b & 2b') penicillinases and cephalosporinases. It varies in its ability to inhibit class II and IV (2a & 4) penicillinases. Tazobactam does not induce chromosomally-mediated beta-lactamases at tazobactam concentrations achieved with the recommended dosage regimen.
Antimicrobial Activity
Piperacillin and tazobactam for injection has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)]:
Aerobic bacteria
Gram-positive bacteria:
Staphylococcus aureus (methicillin susceptible isolates only)
Gram-negative bacteria:
Acinetobacter baumannii
Escherichia coli
Haemophilus influenzae (excluding beta-lactamase negative, ampicillin-
resistant isolates)
Klebsiella pneumoniae
Pseudomonas aeruginosa (given in combination with an aminoglycoside to which
the isolate is susceptible)
Anaerobic bacteria:
Bacteroides fragilis group (B. fragilis, B. ovatus, B. thetaiotaomicron, and
B. vulgatus)
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for piperacillin/tazobactam against isolates of similar genus or organism group. However, the efficacy of piperacillin and tazobactam in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Aerobic bacteria
Gram-positive bacteria
Enterococcus faecalis (ampicillin or penicillin-susceptible isolates only)
Staphylococcus epidermidis (methicillin susceptible isolates only)
Streptococcus agalactiae†
Streptococcus pneumoniae† (penicillin-susceptible isolates only)
Streptococcus pyogenes†
Viridans group streptococci†
Gram-negative bacteria
Citrobacter koseri
Moraxella catarrhalis
Morganella morganii
Neisseria gonorrhoeae
Proteus mirabilis
Proteus vulgaris
Serratia marcescens
Providencia stuartii
Providencia rettgeri
Salmonella enterica
Anaerobic bacteria
Clostridium perfringens
Bacteroides distasonis
Prevotella melaninogenica
†These are not beta-lactamase producing bacteria and, therefore, are susceptible to piperacillin alone.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria, and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
