LensMate Preservative-Free Artificial Tears
001-01
3eda8519-56be-cb15-e063-6394a90a8245
HUMAN OTC DRUG LABEL
Sep 15, 2025
YD BIO USA, INC.
DUNS: 118330659
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lubricant Eye Drops
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
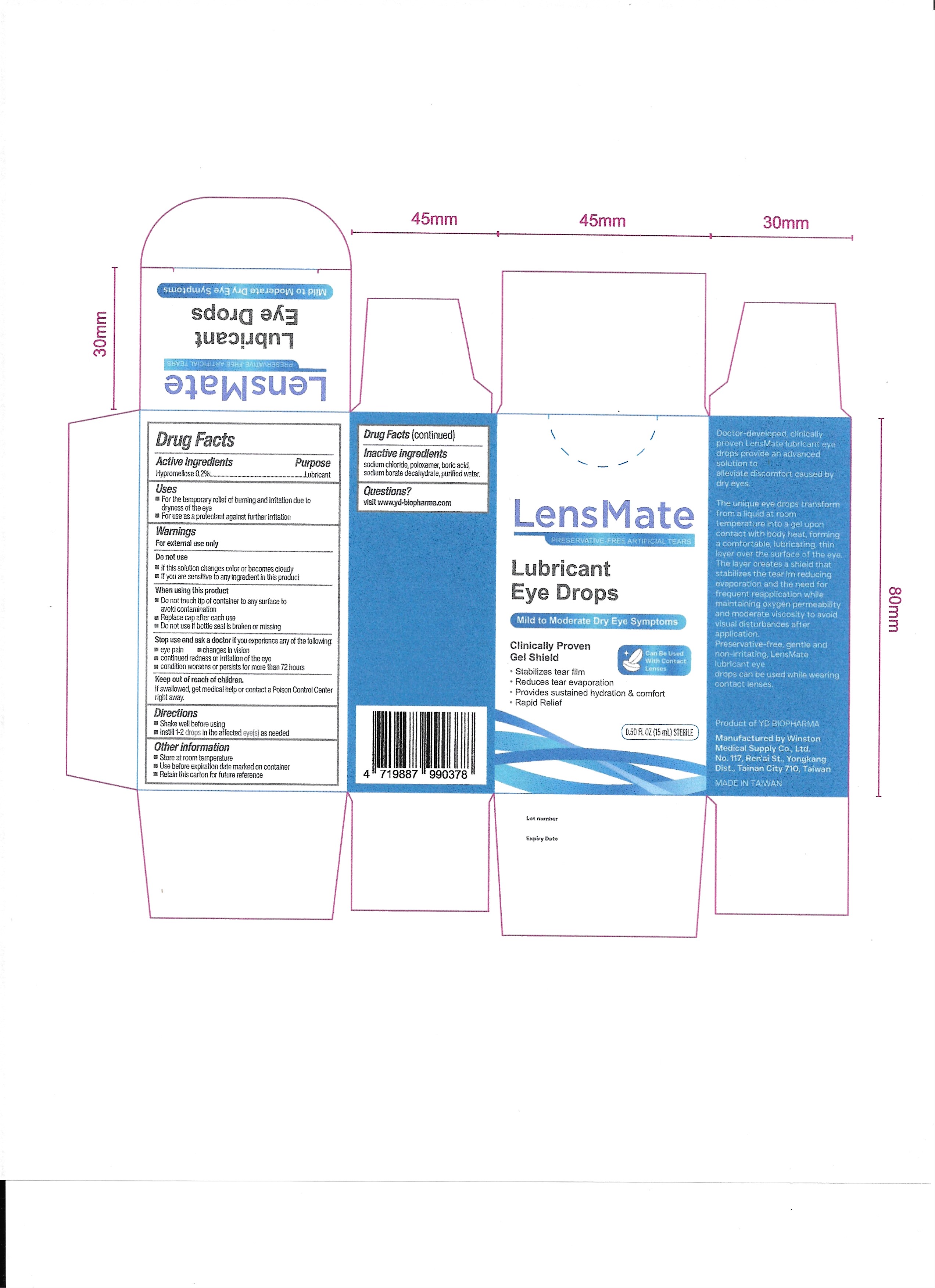

INDICATIONS & USAGE SECTION
For the temporary relief of burning and irritation due to dryness of the eye
For use as a protectant against further irritation
OTC - ACTIVE INGREDIENT SECTION
Hypromellose 0.2%
OTC - PURPOSE SECTION
Lubricant
OTC - ASK DOCTOR SECTION
Stop use and ask a doctor if you experience any of the following:
Eye pain
Changes in vision
Continued redness or irritation of the eye
Condition worsens or persists for more than 72 hours
OTC - DO NOT USE SECTION
If this solution changes color or becomes cloudy
If you are sensitive to any ingredient in this product
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
If swallowed, get medical help or contact a Poison Control Center right away.
OTC - STOP USE SECTION
Stop use and ask a doctor if you experience any of the following:
eye pain
Changes in vision
Continued redness or irritation of the eye
Condition worsens or persists for more than 72 hours
OTC - WHEN USING SECTION
Do not touch tip of container to any surface to avoid contamination
Replace cap after each use
Do not use if bottle seal is broken or missing
OTHER SAFETY INFORMATION
Store at room temperature
Use before expiration date marked on container
Retain this carton for future reference
INACTIVE INGREDIENT SECTION
Sodium chloride, poloxamer, boric acid, sodium borate decahydrate, purified water.
OTC - QUESTIONS SECTION
Visit www.yd-biopharma.com
INSTRUCTIONS FOR USE SECTION
Shake well before using
Instill 1-2 drops in the affected eye(s) as needed
DOSAGE & ADMINISTRATION SECTION
Directions
Shake well before using
Instill 1-2 drops in the affected eye(s) as needed
WARNINGS SECTION
For external use only
