Zenpep
These highlights do not include all the information needed to use safely and effectively. See full prescribing information for . Initial U.S. Approval: 2009
7f16f2d4-8df6-4bd7-ac10-56994fa257b0
HUMAN PRESCRIPTION DRUG LABEL
Feb 28, 2024
Aimmune Therapeutics, Inc.
DUNS: 057562771
Products 8
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
pancrelipase lipase, pancrelipase protease, pancrelipase amylase
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
pancrelipase lipase, pancrelipase protease, pancrelipase amylase
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
pancrelipase lipase, pancrelipase protease, pancrelipase amylase
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
pancrelipase lipase, pancrelipase protease, pancrelipase amylase
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
pancrelipase lipase, pancrelipase protease, pancrelipase amylase
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
pancrelipase lipase, pancrelipase protease, pancrelipase amylase
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
pancrelipase lipase, pancrelipase protease, pancrelipase amylase
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
pancrelipase lipase, pancrelipase protease, pancrelipase amylase
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - Lipase 60,000 USP Units
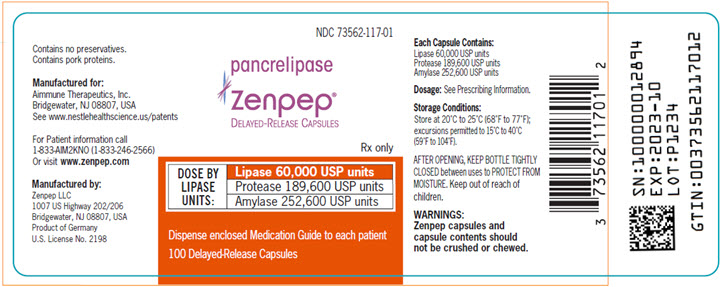
Bottle Label NDC 73562-117-01
NDC 73562-117-01
pancrelipase
Zenpep**®**
Delayed-Release Capsules
Rx only
DOSE BY LIPASE UNITS:
Lipase 60,000 USP units
****Protease 189,600 USP units
Amylase 252,600 USP units
Dispense enclosed Medication Guide to each patient
100 Delayed-Release Capsules
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
ZENPEP® is indicated for the treatment of exocrine pancreatic insufficiency in adult and pediatric patients.
ZENPEP® is indicated for the treatment of exocrine pancreatic insufficiency in adult and pediatric patients. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None
None (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious or otherwise important adverse reactions are described elsewhere in the labeling:
•
Fibrosing Colonopathy [see Warnings and Precautions (5.1)]
•
Irritation of the Oral Mucosa [see Warnings and Precautions (5.2)]
•
Hyperuricemia [see Warnings and Precautions (5.3)]
•
Risk of Viral Transmission [see Warnings and Precautions (5.4)]
•
Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to ZENPEP in 53 adult and pediatric patients with exocrine pancreatic insufficiency due to cystic fibrosis in two clinical trials conducted [see Clinical Studies (14)]. In both trials, ZENPEP was administered at dosages of approximately 5,000 lipase units/kg/day for 19 to 42 days.
Study 1 was a randomized, double-blind, placebo-controlled, crossover study of 34 adult and pediatric patients, aged 7 to 23 years. Adverse reactions that were reported in at least 2 ZENPEP-treated patients (greater than or equal to 6%) and at a higher rate than in placebo-treated patients in Study 1 are shown in Table 1.
Table 1: Adverse Reactions* in a Clinical Trial of Adult and Pediatric Patients 7 Years of Age and Older with Exocrine Pancreatic Insufficiency due to Cystic Fibrosis (Study 1)
| ||
|
Adverse |
ZENPEP |
Placebo |
|
Headache |
5 (15%) |
0 |
|
Contusion |
2 (6%) |
0 |
|
Cough |
2 (6%) |
0 |
|
Early Satiety |
2 (6%) |
0 |
Study 2 was an open-label, uncontrolled study of ZENPEP in 19 pediatric patients aged 1 to 6 years. The most commonly reported adverse reactions were gastrointestinal, including abdominal pain and steatorrhea.
The type and incidence of adverse reactions in Studies 1 and 2 were similar between pediatric patients and adults.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ZENPEP or other pancreatic enzyme products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye Disorders
•
blurred vision
Gastrointestinal Disorders
•
fibrosing colonopathy and distal intestinal obstruction syndrome
•
abdominal distension, abdominal pain, diarrhea, flatulence, constipation, and nausea
Immune System Disorders
•
anaphylaxis, asthma, hives and pruritis
Investigations
•
asymptomatic elevations of liver enzymes
Musculoskeletal System
•
myalgia, muscle spasm
Skin and Subcutaneous Tissue Disorders
•
urticaria and rash
Most common adverse reactions (≥6%) are: headache, contusion, cough, and early satiety. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contactAimmune Therapeutics, Inc at 1-833-AIM2KNO (1-833-246-2566) or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Delayed-release capsules are available in the following strengths:
•
3,000 USP units of lipase; 10,000 USP units of protease; and 14,000 USP units of amylase in a two‑piece hypromellose capsule with a white opaque cap and white opaque body, and red imprint with “APTALIS 3”
•
5,000 USP units of lipase; 17,000 USP units of protease; and 24,000 USP units of amylase in a two‑piece hypromellose capsule with a white opaque cap and white opaque body, and blue imprint with “APTALIS 5”
•
10,000 USP units of lipase; 32,000 USP units of protease; and 42,000 USP units of amylase in a two-piece hypromellose capsule with a yellow opaque cap and white opaque body, and blue imprint with “APTALIS 10”
•
15,000 USP units of lipase; 47,000 USP units of protease; and 63,000 USP units of amylase in a two-piece hypromellose capsule with a red opaque cap and white opaque body, and blue imprint with “APTALIS 15”
•
20,000 USP units of lipase; 63,000 USP units of protease; and 84,000 USP units of amylase in a two-piece hypromellose capsule with a green opaque cap and white opaque body, and blue imprint with “APTALIS 20”
•
25,000 USP units of lipase; 79,000 USP units of protease; and 105,000 USP units of amylase in a two-piece hypromellose capsule with a blue opaque cap and white opaque body, and blue imprint with “APTALIS 25”
•
40,000 USP units of lipase; 126,000 USP units of protease; and 168,000 USP units of amylase in a two-piece hypromellose capsule with an orange opaque cap and white opaque body, and blue imprint with “APTALIS 40”
•
60,000 USP units of lipase; 189,600 USP units of protease; 252,600 USP units of amylase. Capsules have a powder blue opaque cap with two black stripes and white opaque body, black imprint with “APTALIS 60”
Delayed-Release Capsules (3):
•
3,000 USP units of lipase; 10,000 USP units of protease; and 14,000 USP units of amylase
•
5,000 USP units of lipase; 17,000 USP units of protease; and 24,000 USP units of amylase
•
10,000 USP units of lipase; 32,000 USP units of protease; and 42,000 USP units of amylase
•
15,000 USP units of lipase; 47,000 USP units of protease; and 63,000 USP units of amylase
•
20,000 USP units of lipase; 63,000 USP units of protease; and 84,000 USP units of amylase
•
25,000 USP units of lipase; 79,000 USP units of protease; and 105,000 USP units of amylase
•
40,000 USP units of lipase; 126,000 USP units of protease; and 168,000 USP units of amylase
•
60,000 USP units of lipase; 189,600 USP units of protease; and 252,600 USP units of amylase
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published data from case reports with pancrelipase use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Pancrelipase is minimally absorbed systematically; therefore, maternal use is not expected to result in fetal exposure to the drug. Animal reproduction studies have not been conducted with pancrelipase.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of pancrelipase in either human or animal milk, the effects on the breastfed infant or the effects on milk production. Pancrelipase is minimally absorbed systemically following oral administration, therefore maternal use is not expected to result in clinically relevant exposure of breastfed infants to the drug. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZENPEP and any potential adverse effects on the breastfed infant from ZENPEP or from the underlying maternal conditions.
8.4 Pediatric Use
The safety and effectiveness of ZENPEP for the treatment of exocrine pancreatic insufficiency have been established in pediatric patients.
Use of ZENPEP for this indication is supported by an adequate and well- controlled trial in adult and pediatric patients 7 to 17 years of age (Study
- along with supportive data from an open-label, single arm study in 19 pediatric patients 1 to 6 years of age (Study 2). Both study populations consisted of patients with exocrine pancreatic insufficiency due to cystic fibrosis. The safety in pediatric patients in Studies 1 and 2 were similar to that observed in adult patients [see Adverse Reactions (6.1) and Clinical Studies (14)].
Dosages exceeding 6,000 lipase units/kg/meal have been reported postmarketing to be associated with fibrosing colonopathy and colonic strictures in pediatric patients less than 12 years of age. If there is a history of fibrosing colonopathy, monitor patients during treatment with ZENPEP because some patients may be at risk of progressing to stricture formation. Do not exceed the recommended dosage of either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day in pediatric patients greater than 12 months of age without further investigation. [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
Crushing or chewing ZENPEP capsules or mixing the capsule contents in foods having a pH greater than 4.5 can disrupt the protective enteric coating on the capsule contents and result in early release of enzymes, irritation of the oral mucosa, and/or loss of enzyme activity. Instruct the patient or caregiver of the following: consume sufficient liquids (juice, water, breast milk, or formula) to ensure complete swallowing, and visually inspect the mouth of pediatric patients less than 12 months of age to ensure that no drug is retained in the mouth and irritation of the oral mucosa has not occurred [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
8.5 Geriatric Use
Clinical studies of ZENPEP did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between patients aged 65 years and over and younger adult patients.
DESCRIPTION SECTION
11 DESCRIPTION
Pancrelipase is a pancreatic enzyme product consisting of a mixture of enzymes including lipases, proteases, and amylases, and is an extract derived from porcine pancreatic glands. The enteric-coated pellets in ZENPEP are formulated to release pancreatic enzymes at an approximate pH of 5.5 or greater.
ZENPEP (pancrelipase) delayed-release capsule for oral administration, include a two-piece shell containing light brown-colored enteric-coated pellets (1.8 to 1.9mm for 3,000 and 5,000 USP units of lipase, 2.2 to 2.5 mm for 10,000, 15,000, 20,000, 25,000, 40,000 and 60,000 USP units of lipase) and are available as follows:
3,000 USP units of lipase; 10,000 USP units of protease; and 14,000 USP units of amylase; delayed-release capsules have a white opaque cap and a white opaque body with imprint “APTALIS 3”. The shells contain carnauba wax or talc, carrageenan, hypromellose, potassium chloride, titanium oxide, and water. The colorant of the printed ink is red iron oxide.
5,000 USP units of lipase; 17,000 USP units of protease; and 24,000 USP units of amylase; delayed-release capsules have a white opaque cap and a white opaque body with imprint “APTALIS 5”. The shells contain carnauba wax or talc, carrageenan, hypromellose, potassium chloride, titanium oxide, and water. The colorant of the printed ink is FD&C Blue 2.
10,000 USP units of lipase; 32,000 USP units of protease; and 42,000 USP units of amylase; delayed-release capsules have a yellow opaque cap and a white opaque body with imprint “APTALIS 10”. The shells contain carnauba wax or talc, carrageenan, hypromellose, potassium chloride, titanium oxide, water and yellow ferric oxide. The colorant of the printed ink is FD&C Blue 2.
15,000 USP units of lipase; 47,000 USP units of protease; and 63,000 USP units of amylase; delayed-release capsules have a red opaque cap and a white opaque body with imprint “APTALIS 15”. The shells contain carnauba wax or talc, carrageenan, hypromellose, potassium chloride, red ferric oxide, titanium oxide, and water. The colorant of the printed ink is FD&C Blue 2.
20,000 USP units of lipase; 63,000 USP units of protease; and 84,000 USP units of amylase; delayed-release capsules have a green opaque cap and a white opaque body with imprint “APTALIS 20”. The shells contain carnauba wax or talc, carrageenan, FD&C Blue #2, hypromellose, potassium chloride, titanium oxide, water, and yellow ferric oxide. The colorant of the printed ink is FD&C Blue 2.
25,000 USP units of lipase; 79,000 USP units of protease; and 105,000 USP units of amylase; delayed-release capsules have a blue opaque cap and a white opaque body with imprint “APTALIS 25”. The shells contain carnauba wax or talc, carrageenan, FD&C Blue #2, hypromellose, potassium chloride, titanium oxide, and water. The colorant of the printed ink is FD&C Blue 2.
40,000 USP units of lipase; 126,000 USP units of protease; and 168,000 USP units of amylase; delayed-release capsules have an orange opaque cap and white opaque body, printed with “APTALIS 40”. The shells contain FD&C Yellow #6, hypromellose, and titanium oxide. The colorant of the printed ink is FD&C Blue 2.
60,000 USP units of lipase; 189,600 USP units of protease; 252,600 USP units of amylase. Capsules have a powder blue opaque cap with two black stripes and white opaque body, printed with "APTALIS 60" The shells contain FD&C Blue #1, hypromellose, and titanium oxide. The colorant of the printed ink is black iron oxide.
ZENPEP (pancrelipase) delayed-release capsules include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydrogenated castor oil, hypromellose phthalate, magnesium stearate, microcrystalline cellulose, talc, and triethyl citrate.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pancreatic enzyme products contain a mixture of lipases, proteases, and amylases that catalyze the hydrolysis of fats to monoglycerides, glycerol, and free fatty acids, protein into peptides and amino acids, and starch into dextrins and short chain sugars such as maltose and maltriose in the duodenum and proximal small intestine, thereby acting like digestive enzymes physiologically secreted by the pancreas.
12.2 Pharmacodynamics
For patients consuming a high fat diet in the clinical trials, the coefficient of fat absorption (CFA) was higher in patients who received ZENPEP compared to the placebo treatment group, indicating improved fat absorption [see Clinical Studies (14)].
12.3 Pharmacokinetics
Following oral administration, the lipases, proteases, and amylases released from ZENPEP are not absorbed from the gastrointestinal tract in appreciable amounts.
Drug Interactions
The lipases, proteases, and amylases of ZENPEP are not substrates of CYP enzymes or transporters. CYP enzymes or transporters mediated drug interactions are not expected.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Adult and Pediatric Patients 7 Years of Age and Older
Study 1 was a randomized, double-blind, placebo-controlled, crossover study of 34 patients, aged 7 to 23 years, with exocrine pancreatic insufficiency due to cystic fibrosis. The final analysis population was limited to 32 patients, who completed both double-blind treatment periods, and were included in the efficacy analysis population. Patients were randomized to receive ZENPEP or matching placebo for 6 to 7 days of treatment, followed by crossover to the alternate treatment for an additional 6 to 7 days. The mean exposure to ZENPEP during this study, including titration period and open label transition, was 30 days. The mean dosage during the controlled treatment periods ranged from a mean dose of 3,900 lipase units/kg/day to 5,700 lipase units/kg/day. All patients consumed a high-fat diet (greater than or equal to 100 grams of fat per day) during the treatment period. The population was nearly evenly distributed in biological sex, and approximately 96% of patients were White.
Coefficient of Fat Absorption Endpoint and Results
The primary efficacy endpoint was the mean difference in the coefficient of fat absorption (CFA) between ZENPEP and placebo treatment. The CFA was determined by a 72-hour stool collection during both treatments, when both fat excretion and fat ingestion were measured. Each patient’s CFA during placebo treatment was used as their no-treatment CFA value.
Mean CFA was 88% with ZENPEP treatment compared to 63% with placebo treatment. The mean difference in CFA was 26 percentage points in favor of ZENPEP treatment with 95% Confidence Interval of (19, 32) and p≤0.001.
Subgroup analyses of the CFA results showed that mean change in CFA was greater in patients with lower no-treatment (placebo) CFA values than in patients with higher no-treatment (placebo) CFA values. There were similar responses to ZENPEP by age and biological sex.
Pediatric Patients 1 to 6 Years of Age
Study 2 was an open-label, uncontrolled study of 19 pediatric patients, aged 1 to 6 years (mean age 4 years), with exocrine pancreatic insufficiency due to cystic fibrosis. Approximately half of the patients were aged 1 to 3 years. Study 2 compared a measurement of fat malabsorption, spot fecal fat testing, before (while receiving therapy with another pancreatic enzyme product) and after oral administration of ZENPEP capsules with each meal or snack.
After a 4 to 14 day screening period during which patients remained on their current pancreatic enzyme product, patients were switched to ZENPEP at individually titrated dosages ranging between 2,300 and 10,000 lipase units/kg/day (not to exceed 2,500 lipase units/kg/meal) for 14 days. The mean ZENPEP dosage was approximately 5,000 lipase unit/kg/day. There was no wash- out period. Overall, patients showed similar control of fat malabsorption by spot fecal fat testing when switched from their current pancreatic enzyme product to ZENPEP treatment at a similar dosage.
SPL UNCLASSIFIED SECTION
Manufactured by:
****Zenpep LLC
1007 US Highway 202/206,
Bridgewater, NJ 08807, USA
US License No. 2198
Manufactured for:
****Aimmune Therapeutics, Inc.
Bridgewater, NJ 08807, USA
For further information, please call Aimmune Therapeutics at 1-833-AIM2KNO (1-833-246-2566).
©2024 Nestlé
All trademarks are owned by Société des Produits Nestlé S.A., Vevey, Switzerland or used with permission.
Patented. See www.nestlehealthscience.us/patents.
SPL MEDGUIDE SECTION
Medication Guide
|
MEDICATION GUIDE |
|
What is the most important information I should know about ZENPEP? ZENPEP may increase your chance of having a rare bowel disorder called fibrosing colonopathy especially if taken at a high dose for a long time in children with cystic fibrosis. This condition is serious and may require surgery. The risk of having this condition may be reduced by following the dosing instructions that your doctor gave you.Call your doctor right away if you have any unusual or severe: • • • • Take ZENPEP exactly as prescribed by your doctor.Do not take more ZENPEP than directed by your doctor. |
|
What is ZENPEP? • • • |
|
Before taking ZENPEP, tell your doctor about all your medical conditions, including if you: • • • • • • • Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. |
|
How should I take ZENPEP? • • • • • Giving ZENPEP to Adults and Children 12 Months of Age and Older: • • • • • Giving ZENPEP to Infants**(Children from Birth to 12 Months of Age):** The 2 ways to give ZENPEP to infants (children from birth to 12 months of age), which are described below: a) Giving with an Acidic Soft Food b) Giving Directly Into the Infant’s Mouth Before Breastfeeding or Formula Feeding • |
|
What are possible side effects of ZENPEP? ZENPEP may cause serious side effects, including: • • • • The most common side effects of ZENPEP include: • • • • Other Possible Side Effects: ZENPEP and other pancreatic enzyme products are made from the pancreas of pigs, the same pigs people eat as pork. These pigs may carry viruses. Although it has never been reported, it may be possible for a person to get a viral infection from taking pancreatic enzyme products that come from pigs. Tell your doctor if you have any side effect that bothers you or does not go away. These are not all the possible side effects of ZENPEP. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Aimmune Therapeutics, Inc at 1-833-AIM2KNO (1-833-246-2566). |
|
How do I store ZENPEP? • • • • Keep ZENPEP and all medicines out of the reach of children. |
|
General information about the safe and effective use of ZENPEP. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZENPEP for a condition for which it was not prescribed. Do not give ZENPEP to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or doctor for information about ZENPEP that is written for health professionals. |
|
What are the ingredients in ZENPEP? Active ingredients: lipase, protease, amylase Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydrogenated castor oil, hypromellose phthalate, magnesium stearate, microcrystalline cellulose, talc, and triethyl citrate in hypromellose capsules. The red radial imprinting on the 3,000 capsule strength contains, antifoam DC 1510, industrial methylated spirit, iron oxide red C.I. 77491-E172, n-butyl alcohol, shellac and soya lecithin. The blue radial imprinting on the 5,000, 10,000, 15,000, 20,000, 25,000, and 40,000 capsule strengths contains FD&C Blue #2 as colorant. The black radial imprinting on the 60,000 capsule strength contains black iron oxide as colorant. Capsule shell ingredients: • • • • • • • |
|
Manufactured by: Product of Germany US License No. 2198 Manufactured for: For further information, please call Aimmune Therapeutics at 1-833-AIM2KNO (1-833-246-2566) ©2023 Nestlé For further information, please go to www.ZENPEP.com or call Aimmune Therapeutics, Inc at 1-833-AIM2KNO (1-833-246-2566) ©2024 Nestlé All trademarks are owned by Société des Produits Nestlé S.A., Vevey, Switzerland or used with permission. |
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised 02/2024
OVERDOSAGE SECTION
10 OVERDOSAGE
Chronic high dosages of pancreatic enzyme products have been associated with fibrosing colonopathy and colonic strictures [see Warnings and Precautions (5.1)]. High dosages of pancreatic enzyme products have been associated with hyperuricosuria and hyperuricemia [see Warnings and Precautions (5.3)].
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
ZENPEP (pancrelipase) delayed-release capsules containing light, brown-colored delayed-release pancrelipase are supplied as follows:
|
Strength |
Description |
Supplied As |
NDC Number |
|
3,000 USP units of lipase; 10,000 USP units of protease; 14,000 units of amylase |
two-piece hypromellose capsule with white opaque cap and white body with a red radial print and printed with “APTALIS 3” |
Bottles of 100 |
73562-113-01 |
|
5,000 USP units of lipase; 17,000 USP units of protease; 24,000 units of amylase |
two-piece hypromellose capsule with a white opaque cap and white body with a blue radial print and printed with “APTALIS 5” |
Bottles of 100 |
73562-115-01 |
|
10,000 USP units of lipase; 32,000 units of protease; 42,000 units of amylase |
two-piece hypromellose capsule with a yellow opaque cap and white body with a blue radial print and printed with “APTALIS 10” |
Bottles of 100 |
73562-110-01 |
|
15,000 USP units of lipase; 47,000 units of protease; 63,000 units of amylase |
two-piece hypromellose capsule with a red opaque cap and white body with a blue radial print and printed with “APTALIS 15” |
Bottles of 100 |
73562-111-01 |
|
20,000 USP units of lipase; 63,000 units of protease; 84,000 units of amylase |
two-piece hypromellose capsule with a green opaque cap and white body with a blue radial print and printed with “APTALIS 20” |
Bottles of 100 |
73562-112-01 |
|
25,000 USP units of lipase; 79,000 units of protease; 105,000 units of amylase |
two-piece hypromellose capsule with a blue opaque cap and white body with a blue radial print and printed with “APTALIS 25” |
Bottles of 100 |
73562-116-01 |
|
40,000 USP units of lipase; 126,000 units of protease; 168,000 units of amylase |
two-piece hypromellose capsule with an orange opaque cap and white body with a blue radial print and printed with “APTALIS 40” |
Bottles of 100 |
73562-114-01 |
|
60,000 USP units of lipase; 189,600 units of protease; 252,600 units of amylase |
two-piece hypromellose capsule with powder blue opaque cap with two black stripes and white body with a black radial print and printed with “APTALIS 60” |
Bottles of 100 |
73562-117-01 |
Storage and Handling
Original container:
Store at room temperature, 20°C to 25°C (68°F to 77°F) and protect from moisture. Brief excursions permitted to 15°C to 40°C (59°F to104°F) for 24 hours. After opening, keep bottle tightly closed between uses to protect from moisture.
Repackaged HDPE container:
Dispense in tight container (USP). Store at up to 30°C (86°F) for up to 6 months and protect from moisture. Brief excursions permitted to 15°C to 40°C (59°F to 104°F) for up to 30 days. Protect from moisture. After opening, keep bottle tightly closed between uses to protect from moisture.
Zenpep is dispensed in bottles containing a desiccant.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide).
Fibrosing Colonopathy
Advise the patient or caregiver that fibrosing colonopathy has been reported with high dosages of pancreatic enzyme products, usually with use over a prolonged period of time and in pediatric patients with cystic fibrosis. Colonic stricture has been reported in pediatric patients less than 12 years of age. Advise patients and caregivers that if signs and symptoms of colon stricture formation occur (e.g., stomach area (abdominal) pain, bloating, trouble passing stool (constipation), nausea, vomiting, diarrhea) to immediately contact their healthcare provider [see Warnings and Precautions (5.1)].
Hyperuricemia
Advise the patient or caregiver that hyperuricemia may occur in patients with gout or renal impairment and to contact the healthcare provider if they experience pain, stiffness, redness or swelling of their joints [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Inform the patient or caregiver that severe hypersensitivity reactions, including anaphylaxis asthma, hives, and pruritus, have been reported with use of pancreatic enzyme products. Seek medical attention if signs or symptoms of a hypersensitivity reaction develop [see Warnings and Precautions (5.5)].
Dosage
Advise the patient or caregiver to take ZENPEP as prescribed, and to contact the healthcare provider if signs and symptoms of malabsorption persist [see Dosage and Administration (2.2)].
Administration
Instruct the patient or caregiver as follows:
•
Take ZENPEP with meals or snacks.
•
Swallow capsules whole.
•
For adult and pediatric patients who are unable to swallow intact capsules, the capsule contents may be sprinkled on a small amount of acidic soft food with a pH of 4.5 or less (e.g., commercially available preparations of applesauce, bananas, or pears). For pediatric patients birth to 12 months of age, ZENPEP capsules can also be opened, and the capsule contents sprinkled directly into the infant’s mouth.
•
Consume sufficient liquids (juice, water, breast milk, or formula) and visually inspect an infant’s mouth to ensure complete swallowing of ZENPEP capsules or capsule contents [see Warnings and Precautions (5.2)].
•
Do not crush or chew ZENPEP capsules or capsule contents.
•
Do not mix the ZENPEP capsule contents directly into a bottle of breast milk or formula.
Storage
Instruct the patient or caregiver as follows:
•
Keep ZENPEP in a dry place and protect from moisture and heat.
•
After opening, keep bottle tightly closed between uses to protect from moisture.
•
The desiccant packet should not be eaten or thrown away.
