Rifabutin
Rifabutin Capsules, USP Rx only
df7d4103-42e4-4e4f-e053-2a95a90a8ce8
HUMAN PRESCRIPTION DRUG LABEL
Mar 2, 2023
Marlex Pharmaceuticals, Inc.
DUNS: 782540215
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Rifabutin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Rifabutin Capsules for oral administration contain 150 mg of the rifamycin antimycobacterial agent rifabutin, USP, per capsule along with the inactive ingredients, microcrystalline cellulose, sodium lauryl sulfate, colloidal silicon dioxide, magnesium stearate. The hard gelatin capsule contains titanium dioxide, red iron oxide, gelatin, sodium lauryl sulfate and purified water. The imprinting ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide and purified water.
The chemical name for rifabutin is 1',4-didehydro-1-deoxy-1,4-dihydro-5'-(2- methylpropyl)-1-oxorifamycin XIV (Chemical Abstracts Service, 9th Collective Index) or (9 S,12 E,14 S,15 R, 16 S,17 R,18 R,19 R,20 S,21 S,22 E, 24 Z)-6,16,18,20-tetrahydroxy-1'-
isobutyl-14-methoxy- 7,9,15,17,19,21,25-heptamethyl-spiro [9,4- (epoxypentadeca[1,11,13]trienimino)-2 H- furo[2',3':7,8]naphth[1,2-d] imidazole-2,4'- piperidine]-5,10,26-(3 H,9 H)-trione-16-acetate. Rifabutin has a molecular formula of C 46H 62N 4O 11, a molecular weight of 847.02 and the following structure:
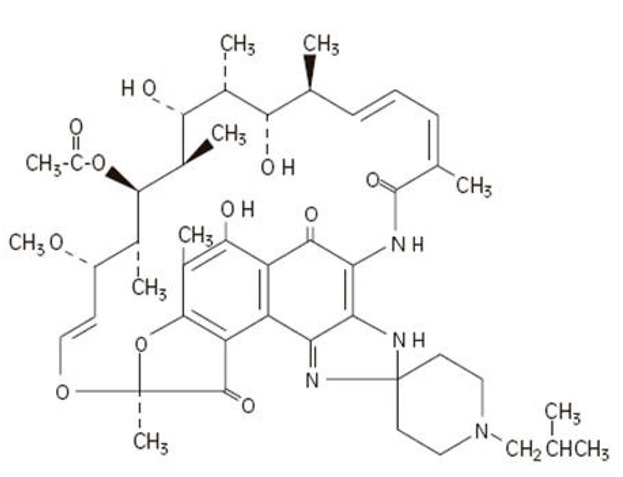
Rifabutin is a red-violet powder soluble in methanol, slightly soluble in ethanol, and slightly soluble in water (0.21 mg/mL). Its log P value (the base 10 logarithm of the partition coefficient between n-octanol and water) is 3.2 (n-octanol/water).
FDA approved dissolution method differs from the current USP monograph dissolution method.
