DESMOPRESSIN ACETATE
These highlights do not include all the information needed to use DESMOPRESSIN NASAL SPRAY safely and effectively. See full prescribing information for DESMOPRESSIN NASAL SPRAY. DESMOPRESSIN nasal spray Initial U.S. Approval: 1978
160fbc1c-81a4-47a9-a814-025f9107e54b
HUMAN PRESCRIPTION DRUG LABEL
Sep 18, 2023
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DESMOPRESSIN ACETATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
DESCRIPTION SECTION
11 DESCRIPTION
Desmopressin nasal spray, USP is a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. The structural formula for the active ingredient is:
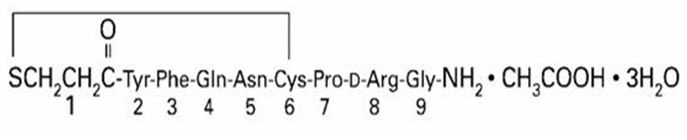
Mol. wt**.**1183.34 g/mol
Molecular formula: C46H64N14O12S2 • C2H4O2 • 3H2O
Chemical name: 1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate.
Desmopressin nasal spray, USP is provided as an aqueous solution for intranasal use.
|
Each mL contains: |
|
Desmopressin acetate 0.1 mg |
|
Inactive: |
|
Benzalkonium chloride solution (50% w/v) 0.2 mg |
|
Citric acid monohydrate 1.7 mg |
|
Sodium chloride 7.5 mg |
|
Sodium phosphate dibasic heptahydrate 4.52 mg |
|
Purified water to 1 mL |
The desmopressin nasal spray, USP compression pump delivers 0.1 mL (10 mcg) of desmopressin acetate per spray.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with desmopressin acetate have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
SPL PATIENT PACKAGE INSERT SECTION
Patient Information
**Desmopressin**Nasal Spray, USP10 mcg per 0.1 mL
(des·mo·pres·sin)
For Intranasal Use Only
What isdesmopressin nasal spray?
Desmopressin nasal spray is a prescription medicine called a vasopressin analog used as an antidiuretic replacement to manage central diabetes insipidus.
- Desmopressin nasal spray is not effective in the treatment of nephrogenic diabetes insipidus.
- Desmopressin nasal spray should not be used in people with nasal problems that may keep them from using a nasal spray.
Who should not use desmopressin nasal spray?
Do not use desmopressin nasal spray if you:
- are allergic to desmopressin or any of the ingredients in desmopressin nasal spray. Some people have had severe allergic reactions while taking desmopressin nasal spray. See the end of this Patient Information for a complete list of ingredients in desmopressin nasal spray.
- have kidney problems
- have or have had low levels of sodium in your blood (hyponatremia)
**What should I tell my healthcare provider before using desmopressin nasal spray?**Before using desmopressin nasal spray, tell your healthcare provider about all your medical conditions including if you:
- have or have had nasal sores, nasal surgery, nasal injury, or have problems such as a stuffy nose or trouble breathing through your nose.
- have or have had any heart, blood circulation, or blood pressure problems
- have a condition that causes fluid or water imbalance problems such as cystic fibrosis, or heart failure.
- have or have had a condition that causes you to be very thirsty
- are pregnant or plan to become pregnant. It is not known if desmopressin will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if desmopressin passes into your breast milk. You and your healthcare provider should decide if you will use desmopressin nasal spray.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use desmopressin nasal spray?
- Read theInstructions for Usethat comes with your desmopressin nasal spray.
- Use desmopressin nasal spray exactly as your healthcare provider tells you to use it.
- Your healthcare provider may change your dose of desmopressin nasal spray if needed.
What are the possible side effects of desmopressin nasal spray?
Desmopressin nasal spray may cause serious side effects including:
***low levels of sodium in the blood (hyponatremia).**People using desmopressin nasal spray are at risk for low sodium levels in the blood, water intoxication, and fluid overload. Follow your healthcare provider’s instructions on limiting the amount of fluid you can drink when using desmopressin nasal spray. *Do notdrink more than you need to satisfy your thirst. You can have serious side effects such as seizures, coma, and death from drinking too much fluid.
- Children and the elderly are at higher risk for these problems and should follow their healthcare provider’s limits on drinking fluids.
- Call your healthcare provider right away if you have any of the following symptoms while using desmopressin nasal spray. They may mean that your blood sodium level is too low.
- headache
- nausea, vomiting
- weight gain
- restlessness
- tiredness
- sleepiness
- disorientation
- loss of appetite
- feeling irritable
- feeling weak
- muscle cramps
- hallucinations
- confusion ***nasal scarring or swelling.**Some people using desmopressin nasal spray for long periods of time may have nasal problems such as scarring or swelling. This may affect how well desmopressin nasal spray works for you. The most common side effects of desmopressin nasal spray include: headache, stuffy nose, runny nose, nosebleed, sore throat, cough, upper respiratory infections, nausea, flushing, and stomach cramps. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use ofdesmopressin nasal spray. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use desmopressin nasal spray for a condition for which it was not prescribed. Do not give desmopressin nasal spray to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about desmopressin nasal spray that is written for health professionals. For more information callApotex Corp. at 1-800-706-5575.
What are the ingredients in desmopressin nasal spray?
**Active ingredient:**desmopressin acetate
Inactive ingredients: benzalkonium chloride solution (50% w/v), citric acid monohydrate, sodium chloride, sodium phosphate dibasic heptahydrate, and purified water
This Patient Information has been approved by the Food and Drug Administration
APOTEX INC.
Desmopressin Nasal Spray, USP10 mcg/ 0.1 mL
|
Manufactured by |
Manufactured for |
September 2022
