Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**4.2 Posology and method of administration** ARTISS is intended for Hospital Use Only by suitably experienced physicians or surgeons. Posology: The amount of ARTISS to be applied and the frequency of application should always be oriented towards the underlying clinical needs of the patient. The dose to be applied is governed by variables including, but not limited to, the type of surgical intervention, the size of the area and the mode of intended application, and the number of applications. Application of the product must be individualised by the treating physician. In clinical trials, the individual dosages have typically ranged from 0.2 – 12 ml. For some procedures (e.g. the sealing of large burned surfaces), larger volumes may be required. The initial amount of the product to be applied at a chosen anatomic site or target surface area should be sufficient to entirely cover the intended application area. The application can be repeated, if necessary, to any small areas that may have not been previously treated. However, avoid reapplication of ARTISS to a pre-existing polymerised ARTISS layer as ARTISS will not adhere to a polymerised layer. As a guideline for the gluing of surfaces, 1 pack of ARTISS 2 ml (i.e., 1 ml Sealer Protein Solution plus 1 ml Thrombin Solution) will be sufficient for an area of at least 10 cm2. When the fibrin sealant is applied by spray application the same quantity will be sufficient to coat an area of up to 100 cm2, depending on the specific indication and the individual case. For large surface areas, spray application is recommended. The required amount of ARTISS depends on the size of the surface to be covered. The approximate surface areas covered by each package size of ARTISS by spray application are: 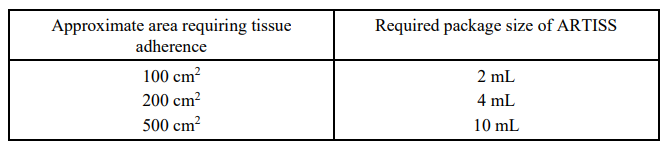 This recommended amount applies to all age groups. The skin graft should be attached to the wound bed immediately after ARTISS has been applied. The surgeon has up to 60 seconds to manipulate and position the graft prior to polymerisation. After the flap or graft has been positioned, hold in the desired position by gentle compression for at least 3 minutes to ensure ARTISS sets properly and the graft or flap adheres firmly to the underlying tissue. To avoid the formation of excess granulation tissue and to ensure gradual absorption of the solidified fibrin sealant, only a thin layer of the mixed Sealer Protein – Thrombin Solution should be applied. ARTISS has not been administered to patients > 65 years old in clinical trials. _Paediatric Population_ Currently available data are described in section 5.1 but no recommendation on a posology can be made – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Method and route of administration For epilesional (topical) use. Do not inject. For subcutaneous use only. ARTISS is not recommended for laparoscopic surgery. See also section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. In order to ensure optimal safe use of ARTISS it should be sprayed using a pressure regulator device that delivers a maximum pressure of up to 2.0 bar (28.5 psi). ARTISS must be sprayed only onto application sites that are visible. ARTISS should only be reconstituted and administered according to the instructions and with the devices recommended for this product (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Prior to applying ARTISS the surface area of the wound needs to be dried by standard techniques (e.g. intermittent application of compresses, swabs, use of suction devices). Do not use pressurized air or gas for drying the sites. For spray application, see sections 4.4 and 6.6 for specific recommendations on the required pressure and distance from tissue per surgical procedure and length of applicator tips – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
TOPICAL
Medical Information
**4.1 Therapeutic indications** ARTISS is indicated to adhere autologous skin grafts to surgically prepared wound beds resulting from burns in adult and paediatric populations greater than or equal to 1 year of age. ARTISS is indicated to adhere tissue flaps during facial rhytidectomy surgery (face-lift) in adults. ARTISS is not indicated for haemostasis.
**4.3 Contraindications** ARTISS is not indicated to replace skin sutures intended to close surgical wounds. ARTISS alone is not indicated for the treatment of massive and brisk arterial or venous bleeding. ARTISS must never be applied intravascularly. Such use has been associated with inadvertent intravascular injection, with thromboembolic complications. ARTISS should only be used topically. Additionally, soft tissue injection of ARTISS carries the risk of an anaphylactic reaction and/or local tissue damage. ARTISS is contraindicated in the case of hypersensitivity to the active substances or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. (see also section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Spray application of ARTISS should not be used in endoscopic procedures. For laparoscopy, see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
B02BC, V031K
xb 02 bc, v 031 k
Manufacturer Information
BAXTER HEALTHCARE (ASIA) PTE LTD
Takeda Manufacturing Austria AG (Site: Industriestrasse 72)
Takeda Manufacturing Austria AG (Site: Lange Allee 24B)
Takeda Manufacturing Austria AG (Site: Lange Allee 24A) (Primary and Secondary Packager)
Active Ingredients
Documents
Package Inserts
Artiss PI - clean.pdf
Approved: December 24, 2021
