Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER, METERED
**4.2 Posology and method of administration** The dosage of Symbicort Turbuhaler should be individualised according to disease severity. The dosage of the components of Symbicort is individual and should be adjusted to the severity of the disease. This should be considered not only when treatment with combination products is initiated but also when the maintenance dose is adjusted. If an individual patient should require a combination of doses other than those available in the combination inhaler, appropriate doses of β2-agonists and/or corticosteroids by individual inhalers should be prescribed. **Asthma** Symbicort can be used according to different treatment approaches: 1. Symbicort anti-inflammatory reliever therapy. 2. Symbicort anti-inflammatory reliever plus maintenance therapy. As an alternative, Symbicort can be used in a fixed dose therapy: 1. Symbicort maintenance therapy. 1. **Symbicort anti-inflammatory reliever therapy (patients with mild disease):** _**Symbicort Turbuhaler 160/4.5 mcg/dose**_ Symbicort is taken as needed for the relief of asthma symptoms when they occur, and to prevent allergen- or exercise-induced bronchoconstriction (or to prevent symptoms in those circumstances recognised by the patient to precipitate an asthma attack). The formoterol component in Symbicort Turbuhaler provides fast onset of effect (within 1–3 minutes) with long-acting (at least 12 hours after a single dose) bronchodilation in reversible airways obstruction. Patients should be advised to always have Symbicort available for relief of symptoms. Clinical studies have demonstrated that Symbicort anti-inflammatory reliever therapy provides significant reductions in severe exacerbations and was statistically superior on daily asthma symptom control compared to a short-acting β2 agonist therapy alone, and comparable to budesonide maintenance therapy given with as-needed short-acting β2 agonist in reducing severe exacerbations (see Section 5.1 Pharmacodynamic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Asthma symptom control was inferior for Symbicort as needed compared to a maintenance dose of corticosteroid given with as needed short-acting β2 agonist. _**Recommended doses:**_ Physicians should discuss allergen exposure and exercise patterns with the patients and take these into consideration when recommending the dose frequency. _**Adults and adolescents (12 years and older):**_ Patients should take 1 inhalation as needed in response to symptoms and for the prevention of allergen- or exercise-induced bronchoconstriction to control asthma. If symptoms persist after a few minutes, 1 additional inhalation should be taken. Not more than 6 inhalations should be taken on any single occasion. A total daily dose of more than 8 inhalations is normally not needed, however a total daily dose of up to 12 inhalations can be used temporarily. Patients using more than 8 inhalations daily should be reassessed for alternative explanations of persisting symptoms. _**Children under 12 years:**_ Efficacy and safety of Symbicort anti-inflammatory reliever therapy in children under 12 years have not been studied. 2. **Symbicort anti-inflammatory reliever plus maintenance therapy:** When maintenance treatment with a combination of inhaled corticosteroid and long-acting β2 agonist is required, Symbicort is taken both as an anti-inflammatory reliever and as regular maintenance treatment. The as-needed inhalations provide both rapid relief of symptoms and improved asthma control. Patients should be advised to have Symbicort available for relief of symptoms at all times. A separate reliever inhaler is not necessary. Clinical studies have demonstrated that Symbicort anti-inflammatory reliever plus maintenance therapy provides clinically meaningful reductions in severe exacerbations while maintaining symptom control, compared to Symbicort maintenance therapy with a separate short-acting bronchodilator (see Section 5.1 Pharmacodynamic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Recommended doses:**_ When control has been achieved, the dose should be titrated to the lowest dose at which effective control of symptoms is maintained.  3. **Symbicort maintenance therapy (fixed dose):** When maintenance treatment with a combination of inhaled corticosteroid and long-acting β2 agonist is required, Symbicort is taken as a fixed daily dose treatment, with a separate short-acting bronchodilator as reliever. Patients should be advised to have their separate short-acting bronchodilator available for relief of symptoms at all times. _**Recommended doses:**_ When control has been achieved, the dose should be titrated to the lowest dose at which effective control of symptoms is maintained. Increasing use of a separate rapid-acting bronchodilator indicates a worsening of the underlying condition and warrants a reassessment of the asthma therapy. 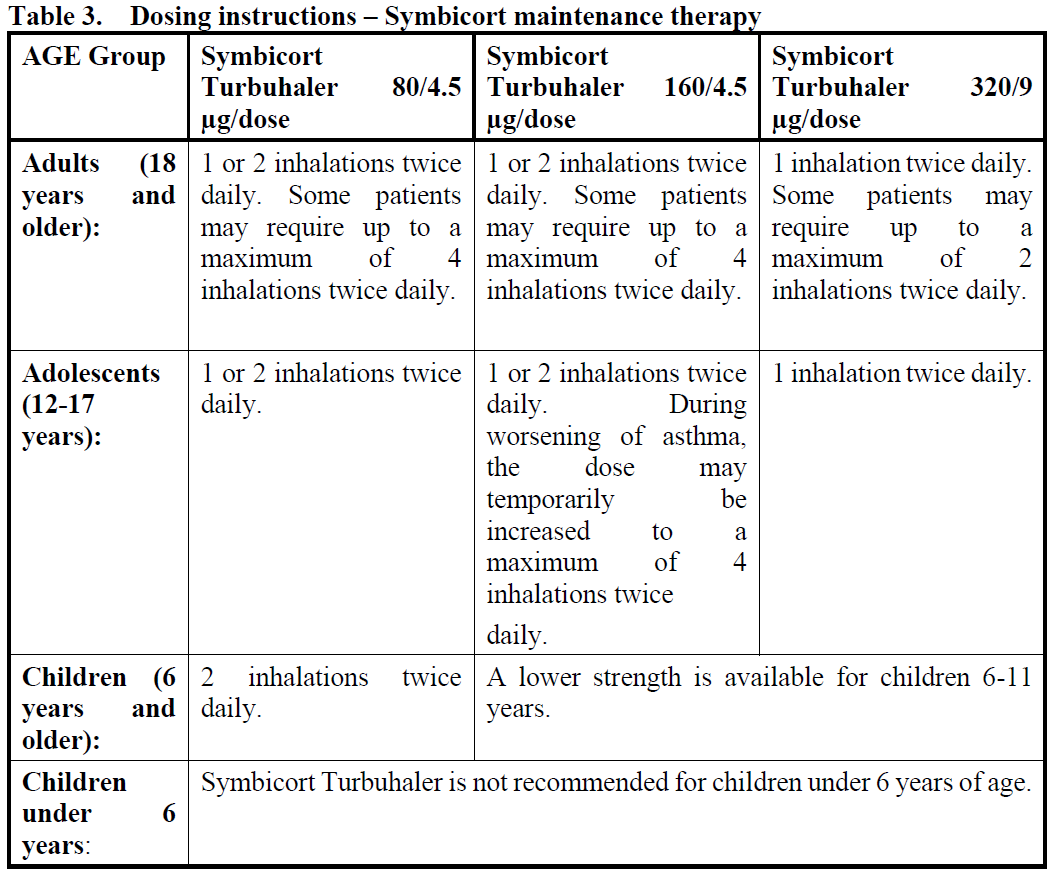 **COPD** 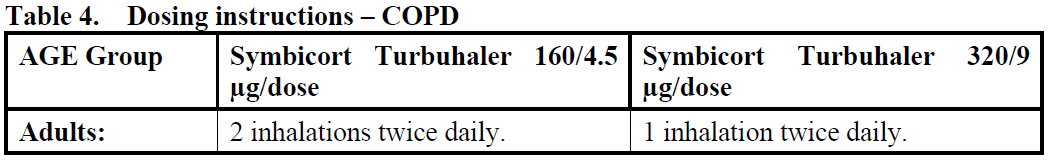 **General information** If patients take Symbicort as a maintenance therapy, they should be instructed that Symbicort Turbuhaler must be used even when asymptomatic for optimal benefit. _**Special patient groups:**_ There are no special dosing requirements for elderly patients. There are no data available for use of Symbicort Turbuhaler in patients with hepatic or renal impairment. As budesonide and formoterol are primarily eliminated via hepatic metabolism, an increased exposure can be expected in patients with severe liver cirrhosis. **Instructions for correct use of Symbicort Turbuhaler:** Turbuhaler is inspiratory flow-driven, which means that when the patient inhales through the mouthpiece, the substance will follow the inspired air into the airways. **Note:** It is important to instruct the patient - To carefully read the instructions for use/handling written at the end of this leaflet. - To breathe in forcefully and deeply through the mouthpiece to ensure that an optimal dose is delivered to the lungs. - Never to breathe out through the mouthpiece. - To replace the cover of the Symbicort Turbuhaler after use. - Rinse the mouth with water after inhaling the maintenance dose to minimise the risk of oropharyngeal thrush. The patient may not taste or feel any medication when using Symbicort Turbuhaler due to the small amount of drug dispensed.
RESPIRATORY (INHALATION)
Medical Information
**4.1 Therapeutic indications** 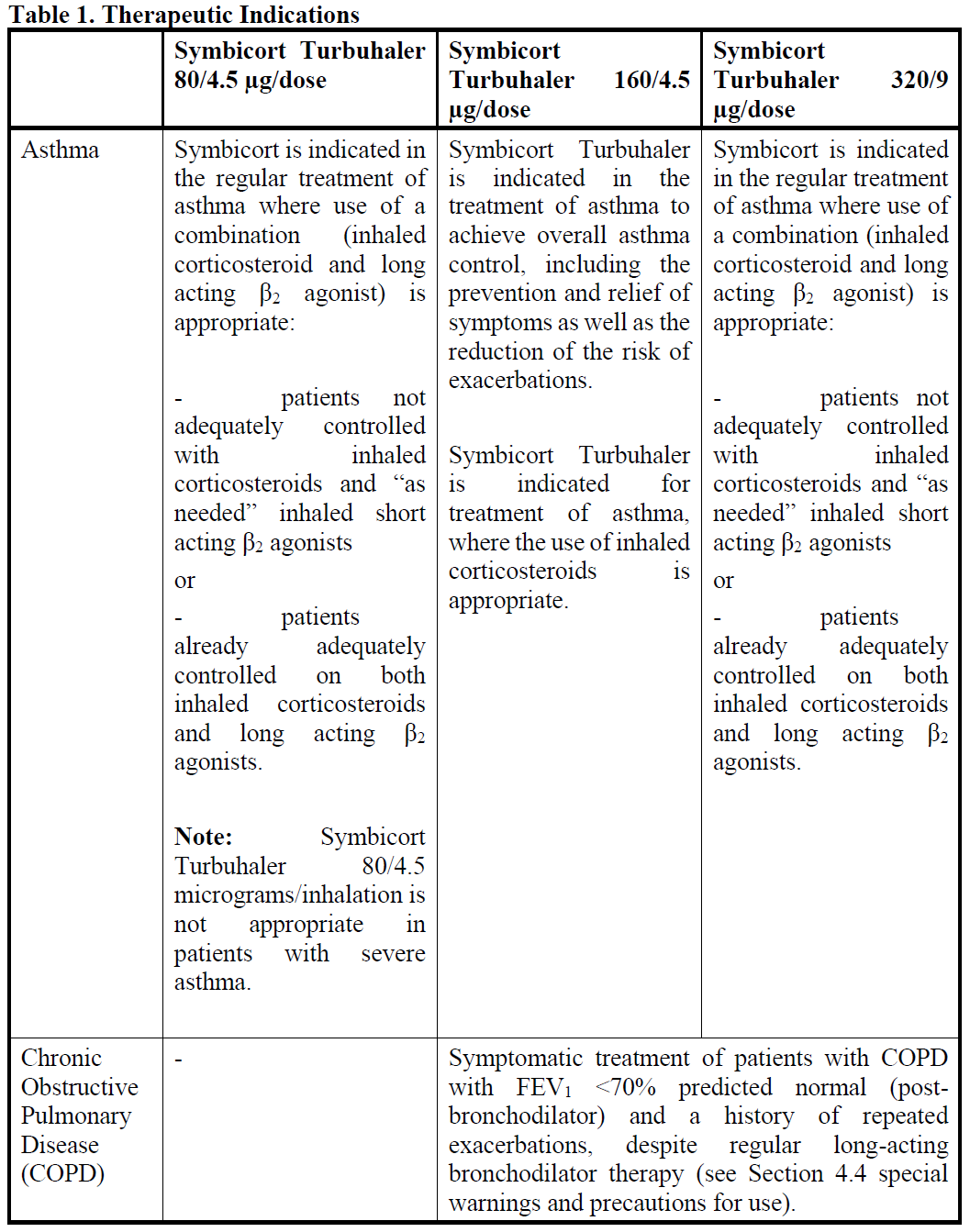
**4.3 Contraindications** Hypersensitivity (allergy) to budesonide, formoterol or lactose (which contains small amounts of milk proteins).
R03AK07
formoterol and budesonide
Manufacturer Information
ASTRAZENECA SINGAPORE PTE LTD
ASTRAZENECA AB
Active Ingredients
Documents
Package Inserts
Symbicort Turbuhaler PI.PDF
Approved: September 15, 2022
