Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2 DOSAGE AND ADMINISTRATION** **2.1 Recommended Dosage** The recommended dosage of Lonsurf® is 35 mg/m2 up to a maximum of 80 mg per dose (based on the trifluridine component) orally twice daily within one hour of completion of morning and evening meals on Days 1 through 5 and Days 8 through 12 of each 28-day cycle until disease progression or unacceptable toxicity. Round dose to the nearest 5 mg increment. Instruct patients to swallow Lonsurf® tablets whole. Instruct patients not to retake doses of Lonsurf® that are vomited or missed and to continue with the next scheduled dose. Table 1 shows the calculated initial daily dose based on body surface area (BSA). 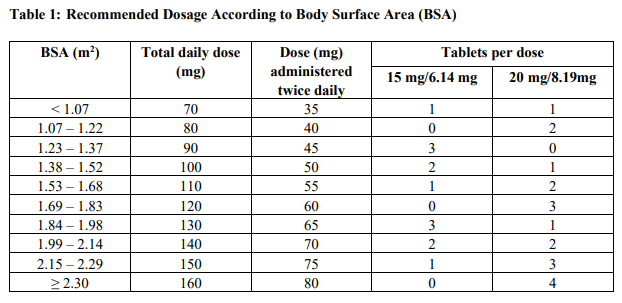 **2.2 Dosage Modifications for Adverse Reactions** Obtain complete blood cell counts prior to and on Day 15 of each cycle. _\[see Warnings and Precautions (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ Do not initiate the cycle of Lonsurf® until: - Absolute neutrophil count (ANC) is greater than or equal to 1,500/mm3 or febrile neutropenia is resolved - Platelets are greater than or equal to 75,000/mm3 - Grade 3 or 4 non-hematological adverse reactions are resolved to Grade 0 or 1 Within a treatment cycle, withhold Lonsurf® for any of the following: - Absolute neutrophil count (ANC) less than 500/mm3 or febrile neutropenia - Platelets less than 50,000/mm3 - Grade 3 or 4 non-hematological adverse reaction After recovery, resume Lonsurf® after reducing the dose by 5 mg/m2/dose from the previous dose, if the following occur: - Febrile neutropenia - Uncomplicated Grade 4 neutropenia (which has recovered to greater than or equal to 1,500/mm3) or thrombocytopenia (which has recovered to greater than or equal to 75,000/mm3) that results in more than 1 week delay in start of next cycle - Non-hematologic Grade 3 or Grade 4 adverse reaction except for Grade 3 nausea and/or vomiting controlled by antiemetic therapy or Grade 3 diarrhea responsive to antidiarrheal medication A maximum of 3 dose reductions are permitted. Permanently discontinue Lonsurf® in patients who are unable to tolerate a dose of 20 mg/m2 orally twice daily. Do not escalate Lonsurf® dosage after it has been reduced. **2.3 Recommended Dosage for Renal Impairment** Severe Renal Impairment In patients with severe renal impairment \[creatinine clearance (CLcr) of 15 to 29 mL/min as determined by the Cockcroft-Gault formula\], the recommended initial dosage is 20 mg/m2 (based on the trifluridine component) orally twice daily within one hour of completion of morning and evening meals on Days 1 through 5 and Days 8 through 12 of each 28-day cycle (Table 2). _\[see Use in Specific Populations (7.7), Clinical Pharmacology (10.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ Reduce dose to 15 mg/m2 twice daily in patients with severe renal impairment who are unable to tolerate a dose of 20 mg/m2 twice daily (Table 2). Permanently discontinue Lonsurf® in patients who are unable to tolerate a dose of 15 mg/m2 twice daily. 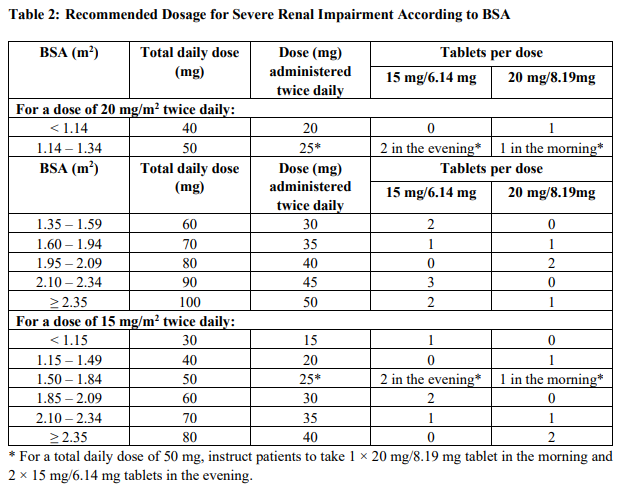
ORAL
Medical Information
**1 INDICATIONS AND USAGE** **1.1 Metastatic Colorectal Cancer** Lonsurf® is indicated for the treatment of adult patients with metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy. **1.2 Metastatic Gastric Cancer** Lonsurf® is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma who have been previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neutargeted therapy.
**4 CONTRAINDICATIONS** None.
L01BC59
trifluridine, combinations
Manufacturer Information
TAIHO PHARMA ASIA PACIFIC PTE. LTD.
Taiho Pharmaceutical Co., Ltd. Kitajima Plant
Active Ingredients
Documents
Package Inserts
Lonsurf PI.pdf
Approved: March 31, 2021
