Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CREAM
**Posology and method of administration** Adults _Intact skin:_ 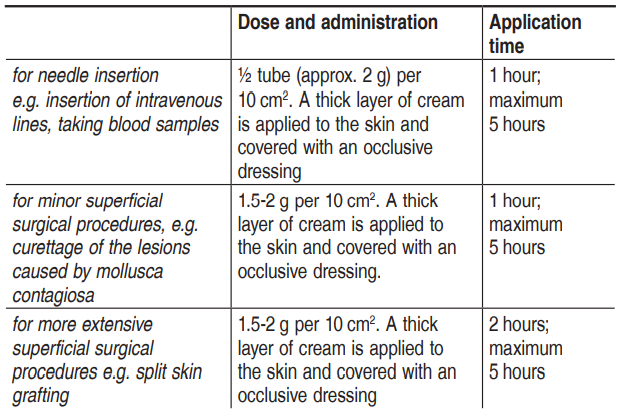 _Leg ulcers:_ For cleaning of leg ulcers: approx. 1–2 g per 10 cm2. The cream is applied in a thick layer to the surface of the ulcer, but not more than 10 g per treatment procedure. Cover the surface of the ulcer with an occlusive dressing. An opened tube is intended for a single use, and any remaining cream must therefore be discarded after each treatment procedure. Application time: at least 30 minutes. For leg ulcers with tissue that is particularly difficult to penetrate the application time may be extended to 60 minutes. Cleaning of the ulcer should begin within 10 minutes after the cream has been removed. EMLA has been used for up to 15 treatment procedures over a period of 1–2 months without a decline in effect or an increase in the number of local reactions. _Genital use_ Skin: Use prior to injection of local anaesthetics: Men: 1 g per 10 cm2. A thick layer of cream is applied to the skin. Application time: 15 minutes. Women: 1–2 g per 10 cm2. A thick layer of cream is applied to the skin. Application time: 60 minutes. Genital mucosa: For removal of condyloma or prior to injection of local anaesthetics: approx. 5–10 g, depending on the area to be treated. The whole surface, including the mucosal folds, must be covered. Occlusion is not necessary. Application time: 5–10 minutes. The surgery must be begun immediately after removal of the cream. Children _For needle insertion, curettage of the lesions caused by mollusca contagiosa and other minor surgical procedures:_ 1 g per 10 cm2. A thick layer of cream is applied to the skin and covered with an occlusive dressing. The dose should not exceed 1 gram per 10 cm2 and must be adjusted according to the application area: 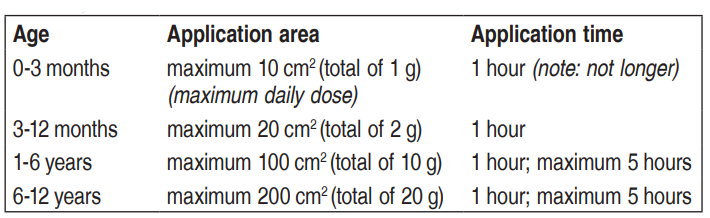 After a longer application time, the anaesthesia decreases. Children with atopic dermatitis: reduce application time to 30 minutes.
TOPICAL
Medical Information
**Therapeutic indication** Surface anaesthesia of the skin in connection with needle insertion and for superficial surgical procedures. Surface anaesthesia of leg ulcers prior to cleaning and superficial surgical procedures, for example removal of fibrin, pus and necroses. Surface anaesthesia of the genital mucosa.
**Contraindications** Known hypersensitivity to local anaesthetics of the amide type or to any of the excipients. EMLA must not be used in premature infants (born before week 37 of pregnancy).
N01BB20
combinations
Manufacturer Information
DCH AURIGA SINGAPORE
Recipharm Karlskoga AB
Aspen Bad Oldesloe GmbH
Active Ingredients
Documents
Package Inserts
Emla Cream PI (Recipharm).pdf
Approved: November 21, 2018
