Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**3.2 Posology and method of administration** 75 mg doses can be administered as either - one 75 mg capsule or - one 30 mg capsule plus one 45 mg capsule During situations when commercially manufactured oseltamivir phosphate capsules oral suspension is not readily available, adults, adolescents or children who are unable to swallow capsules may receive appropriate doses of oseltamivir phosphate capsules by opening capsules and pouring the contents of capsules into a suitable, small amount (1 teaspoon maximum) of sweetened food product such as regular or sugar-free chocolate syrup, honey (only for children two years or older), light brown or table sugar dissolved in water, dessert toppings, sweetened condensed milk, apple sauce or yogurt to mask the bitter taste. The mixture should be stirred and the entire contents given to the patient. The mixture must be swallowed immediately after its preparation. _Treatment of influenza_ Treatment should be initiated as soon as possible within the first two days of onset of symptoms of influenza. For adolescents (13 to 17 years of age) and adults: The recommended oral dose is 75 mg oseltamivir twice daily for 5 days. For infants older than 1 year of age and for children 2 to 12 years of age: The recommended dose of oseltamivir phosphate capsules is indicated in the table below. The following weight-adjusted dosing regimens are recommended: 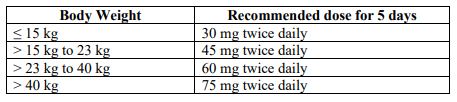 For children 6 to 12 months of age: Depending on the pathogenicity of the circulating influenza virus strain, children between 6 and 12 months of age can be treated with oseltamivir phosphate capsules during a pandemic influenza outbreak, although the available data are limited. Pharmacokinetic data indicate that a dosage of 3 mg/kg twice daily in children 6 to 12 months of age provides plasma drug exposures in the majority of patients similar to those shown to be clinically efficacious in children age one or older and adults (see section 4.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended dose for treatment of children 6 to 12 months of age is 3 mg per kg body weight twice daily for 5 days for treatment. _Prevention of influenza_ _Post-exposure prevention_ For adolescents (13 to 17 years of age) and adults: The recommended dose for prevention of influenza following close contact with an infected individual is 75 mg oseltamivir once daily for 10 days. Therapy should begin as soon as possible within two days of exposure to an infected individual. For infants older than 1 year of age and for children 2 to 12 years of age: The recommended post-exposure prevention dose of oseltamivir phosphate capsules is: 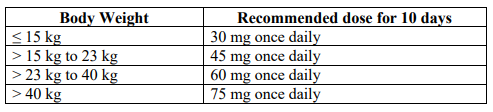 _Prevention during an influenza epidemic in the community_ The recommended dose for prevention of influenza during a community outbreak is 75 mg oseltamivir once daily for up to 6 weeks. _Infants 6–12 months of age_ This procedure describes the preparation of a 10 mg/ml solution that will provide one patient with enough medication for a 5-day course of treatment or a 10-day course of prophylaxis. The pharmacist may compound a suspension (10 mg/ml) from oseltamivir phosphate capsules 30 mg, 45 mg or 75 mg capsules using water containing 0.1% w/v sodium benzoate added as a preservative. First, calculate the Total Volume needed to be compounded and dispensed for each patient. The Total Volume required is determined by the weight of the patient according to the recommendation in the table below: 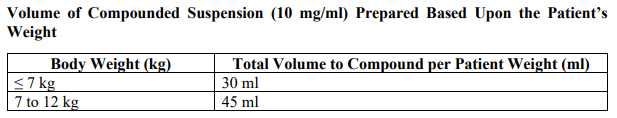 Second, determine the number of capsules and the amount of vehicle (water containing 0.1% w/v sodium benzoate added as a preservative) that is needed to prepare the Total Volume (calculated from the table above: 30 ml, 45 ml) of compounded suspension (10 mg/ml) as shown in the table below: 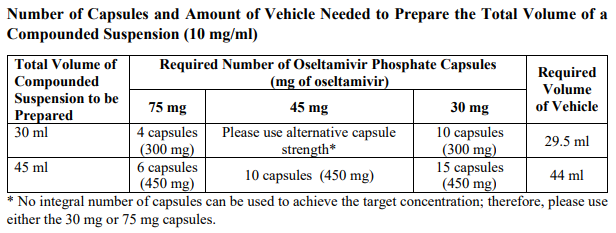 Third, follow the procedure below for compounding the suspension (10 mg/ml) from oseltamivir phosphate capsules: 01. Carefully separate the capsule body and cap and transfer the contents of the required number of oseltamivir phosphate capsules into a clean mortar. 02. Triturate the granules to a fine powder. 03. Add one-third (1/3) of the specified amount of vehicle and triturate the powder until a uniform suspension is achieved. 04. Transfer the suspension to an amber glass or amber polyethyleneterephthalate (PET) bottle. A funnel may be used to eliminate any spillage. 05. Add another one-third (1/3) of the vehicle to the mortar, rinse the pestle and mortar by a triturating motion and transfer the vehicle into the bottle. 06. Repeat the rinsing (Step 5) with the remainder of the vehicle. 07. Close the bottle using a child-resistant cap. 08. Shake well to completely dissolve the active drug and to ensure homogeneous distribution of the dissolved drug in the resulting suspension. (Note: Undissolved residue may be visible but is comprised of inert ingredients of oseltamivir phosphate capsules, which are insoluble. However, the active drug, oseltamivir phosphate, readily dissolves in the specified vehicle and therefore forms a uniform solution.) 09. Put an ancillary label on the bottle indicating “Shake Gently Before Use”. 10. Instruct the parent or caregiver that after the patient has completed the full course of therapy any remaining solution must be discarded. It is recommended that this information be provided by affixing an ancillary label to the bottle or adding a statement to the pharmacy label instructions. 11. Place an appropriate expiration date label according to storage condition (see below). Storage of the pharmacy-compounded suspension (10 mg/ml) Room temperature storage conditions: Stable for 3 weeks (21 days) when stored at room temperature “do not store above 25 °C”. Refrigerated storage conditions: Stable for 6 weeks when stored at 2 °C – 8 °C. Place a pharmacy label on the bottle that includes the patient’s name, dosing instructions, use by date, drug name and any other required information to be in compliance with local pharmacy regulations. Refer to the table below for the proper dosing instructions. 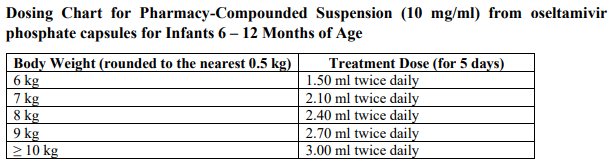 Note: This compounding procedure results in a 10 mg/ml suspension. Dispense the suspension with a graduated oral syringe for measuring small amounts of suspension. If possible, mark or highlight the graduation corresponding to the appropriate dose on the oral syringe for each patient. The appropriate dose must be mixed by the caregiver with an equal quantity of sweet liquid food, such as sugar water, chocolate syrup, cherry syrup, dessert toppings (like caramel or fudge sauce) to mask the bitter taste. **Special populations** _Hepatic impairment_ No dose adjustment is required either for treatment or for prevention in patients with hepatic dysfunction. No studies have been carried out in paediatric patients with hepatic disorder. _Renal impairment_ _Treatment of influenza_: Dose adjustment is recommended for adults and adolescents (13 to 17 years of age) with moderate or severe renal impairment. Recommended doses are detailed in the table below. 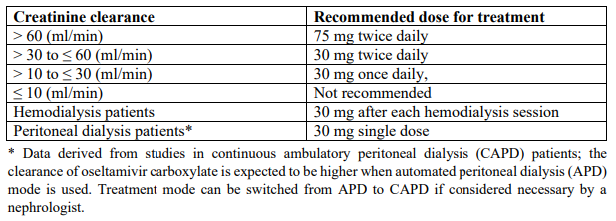 _Prevention of influenza_: Dose adjustment is recommended for adults and adolescents (13 to 17 years of age) with moderate or severe renal impairment as detailed in the table below. 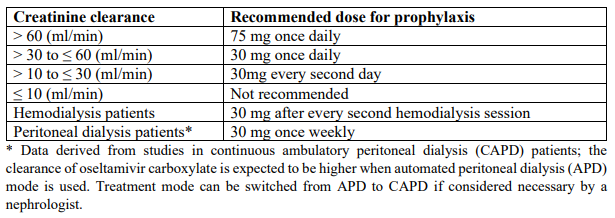 _Geriatric Use_ No dose adjustment is required, unless there is evidence of severe renal impairment. _Children_ There is insufficient clinical data available in children with renal impairment to be able to make any dosing recommendation.
ORAL
Medical Information
**3.1 Therapeutic indications** _Treatment of influenza_ In patients one year of age and older who present with symptoms typical of influenza, when influenza virus is circulating in the community. Efficacy has been demonstrated when treatment is initiated within two days of first onset of symptoms. This indication is based on clinical studies of naturally occurring influenza in which the predominant infection was influenza A (see section 4.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Based on limited pharmacokinetic and safety data, oseltamivir phosphate capsules can be used in children 6 to 12 months of age for treatment during a pandemic influenza outbreak. The treating physician should take into account the pathogenicity of the circulating strain and the underlying condition of the patient to ensure there is a potential benefit to the child. _Prevention of influenza_ - Post-exposure prevention in individuals one year of age or older following contact with a clinically diagnosed influenza case when influenza virus is circulating in the community. - The appropriate use of oseltamivir phosphate capsules for prevention of influenza should be determined on a case by case basis by the circumstances and the population requiring protection. In exceptional situations (e.g., in case of a mismatch between the circulating and vaccine virus strains, and a pandemic situation) seasonal prevention could be considered in individuals one year of age or older. Oseltamivir phosphate capsules are not a substitute for influenza vaccination. The use of antivirals for the treatment and prevention of influenza should be determined on the basis of official recommendations. Decisions regarding the use of antivirals for treatment and prophylaxis should take into consideration what is known about the characteristics of the circulating influenza viruses and the impact of the disease in different geographical areas and patient populations.
**3.3 Contraindications** Oseltamivir phosphate capsules are contraindicated in patients with known hypersensitivity to Oseltamivir phosphate or to any component of the product.
J05AH02
oseltamivir
Manufacturer Information
NATCO PHARMA ASIA PTE. LTD.
NATCO PHARMA LIMITED
Active Ingredients
Documents
Package Inserts
Natflu Capsules PI.pdf
Approved: June 21, 2021
