Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER
Dosage and administration To be taken at the first sign of diarrhoea. 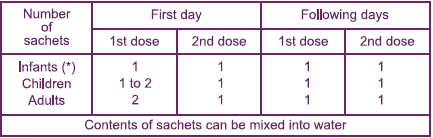 The 2nd dose can be taken 6–8 hours after the 1st dose.
ORAL
Medical Information
Indications Symptomatic treatment of diarrhoea.
A07FA01
lactic acid producing organisms
Manufacturer Information
AMSCO HEALTHCARE MARKETING PTE. LTD.
ADARE PHARMACEUTICALS SAS
Active Ingredients
Documents
Package Inserts
Lacteol Fort Sachet PI.pdf
Approved: October 16, 2018
