Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**【DOSAGE AND ADMINISTRATION】** 1. Usually for adults, administer 250~500mg at meal intervals twice a day. In severe mixed infections, administer up to 750mg twice a day. In acute infections, usually treat for 5 to 10 days. After disappearance of symptoms, further administration is required at least for 3 days. 2. In patients with severe renal disorder, it should be administered according to the following dosage and administration. 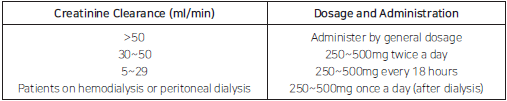 When only the concentration of serum creatinine clearance is known, the patient’s creatinine clearance can be estimated by using the following formulas: 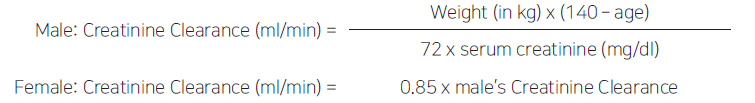 In patient with severe cardiac failure and infections, 750mg can be administered once, but careful monitoring is required and the ciprofloxacin concentration should be periodically estimated. At that time, the peak serum concentration (obtained 1~2 hours after administration) should range from 2~4mcg/ml. 3. Dosage may be adjusted according to causative organisms and severity of symptom.
ORAL
Medical Information
**【INDICATIONS】** 1. Susceptible organisms : E. coli, Shigella, Salmonella, Citrobacter, Klebsiella, Enterobacter, Serratia, hafnia, proteus (Indole positive & negative). Pseudomonas, Neisseria, Acinetobacter, Streptococcus, Chlamydia, Staphylococcus, Corynebacterium, Bacteroides, Clostridium 2. Indications Respiratory infections, infections of oral cavity, teeth and jaw, infections of ear, nose and throat, infections of kidney or urinary passages, genital infections containing gonorrhea, gastrointestinal infections, infection and wound of soft tissue, infections of bone and joint, infections in gynecological and obstetric field, septicemia, cerebromeningitis, peritonitis, ophthalmologic infections and infections in bile secretory duct.
**【PRECAUTIONS】** 1. Contraindications 1. Patients previously found hypersensitive to it. 2. Pregnancy and nursing woman 3. Children and infants 4. Patients with hepatic disorder
J01MA02
ciprofloxacin
Manufacturer Information
ZYFAS PHARMA PTE. LTD.
SHIN POONG PHARMACEUTICAL CO LTD
Active Ingredients
Documents
Package Inserts
Qupron PI.pdf
Approved: May 7, 2021
