Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**4.2 Posology and method of administration** Confirm the presence of an NTRK gene fusion in a tumor specimen prior to initiation of treatment with VITRAKVI. **4.2.1 Dosage regimen** _Adults_ The recommended dose of VITRAKVI in adults is 100 mg taken orally, twice daily until disease progression or until unacceptable toxicity occurs. _Pediatric_ Dosing in pediatric patients is based on body surface area (BSA). The recommended dose of VITRAKVI in pediatric patients is 100 mg/m2 taken orally, twice daily with a maximum of 100 mg per dose until disease progression or until unacceptable toxicity occurs. _Missed dose_ If a dose is missed, the patient should not take two doses at the same time to make up for a missed dose. Patients should take the next dose at the next scheduled time. If the patient vomits after taking a dose, the patient should not take an additional dose to make up for vomiting. _Dose modification_ For all grade 2 adverse reactions, continued dosing may be appropriate, though close monitoring to ensure no worsening of the toxicity is advised. For all grade 3 or 4 adverse reactions not referring to liver function test abnormalities: - VITRAKVI should be withheld until the adverse reaction resolves or improves to baseline or grade 1. Resume at the next dosage modification if resolution occurs within 4 weeks. - VITRAKVI should be permanently discontinued if an adverse reaction does not resolve within 4 weeks. The recommended dosage modifications for VITRAKVI for adverse reactions are provided in Table 1. 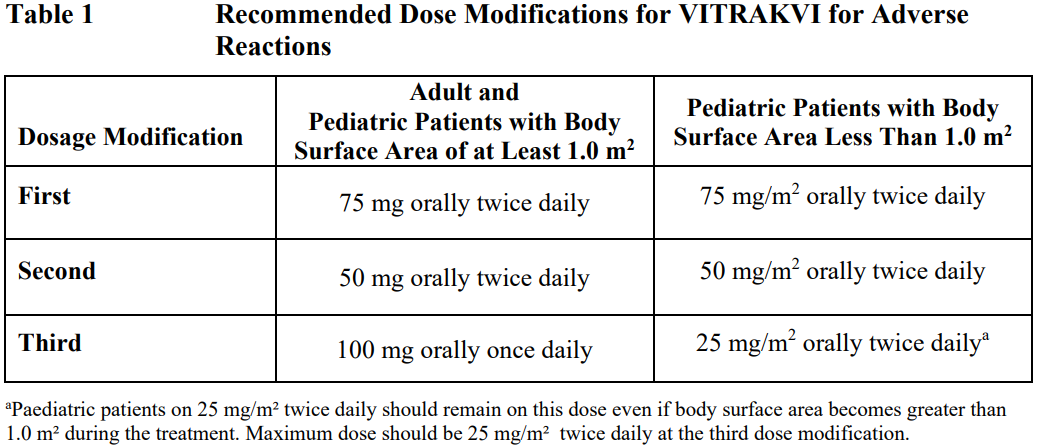 VITRAKVI should be permanently discontinued in patients who are unable to tolerate VITRAKVI after three dose modifications. The recommended dose modifications in case of liver function tests abnormalities during treatment with VITRAKVI are provided in Table 2. 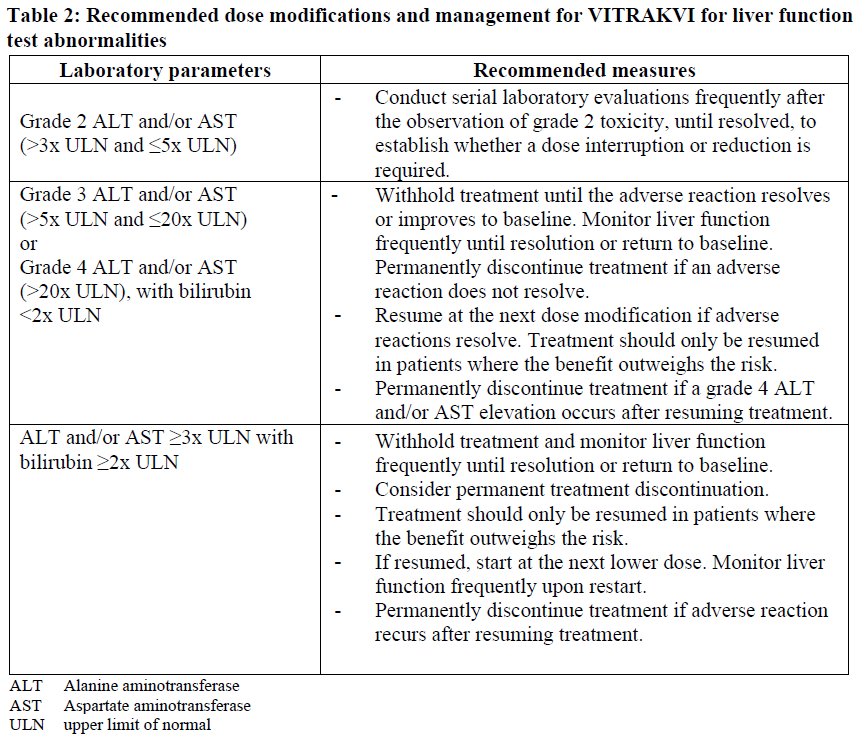 **Special populations** _Elderly_ No dose adjustment is necessary in elderly patients (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ The starting dose of VITRAKVI should be reduced by 50% in patients with moderate (Child-Pugh B) to severe (Child-Pugh C) hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No dose adjustment is required in patients with mild (Child-Pugh A) hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ No dose adjustment is required for patients with renal impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Co-administration with Strong CYP3A4 Inhibitors_ If coadministration of a strong CYP3A4 inhibitor cannot be avoided, reduce the VITRAKVI dose by 50%. After the inhibitor has been discontinued for 3 to 5 elimination half-lives, resume the VITRAKVI dose taken prior to initiating the CYP3A4 inhibitor (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of administration VITRAKVI is for oral use. VITRAKVI is available as a capsule or oral solution with equivalent oral bioavailability and may be used interchangeably. Capsule The patient should be advised to swallow the capsule whole with a glass of water. Due to the bitter taste, the capsule should not be opened, chewed or crushed. The capsules can be taken with or without food but should not be taken with grapefruit or grapefruit juice. Oral Solution The oral solution should be administered by mouth using an oral syringe of 1 mL or 5 mL volume or enterally by using a nasogastric feeding tube. - For doses below 1 mL a 1 mL oral syringe should be used. The calculated dose volume should be rounded to the nearest 0.1 mL. - For doses of 1 mL and higher a 5 mL oral syringe should be used. The dose volume should be calculated to the nearest 0.2 mL. - VITRAKVI should not be mixed with feeding formulas, if administered via nasogastric feeding tube. Mixing with the feeding formulas could lead to tube blockages. The oral solution can be taken with or without food but should not be taken with grapefruit or grapefruit juice.
ORAL
Medical Information
**4.1 Therapeutic indication(s)** VITRAKVI as monotherapy is indicated for the treatment of adult and pediatric patients with solid tumors - that display a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known acquired resistance mutation, - who have a disease that is locally advanced, metastatic or where surgical resection is likely to result in severe morbidity, and - who have no satisfactory treatment options
**4.3 Contraindications** Hypersensitivity to the active substance or any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01EX12
larotrectinib
Manufacturer Information
BAYER (SOUTH EAST ASIA) PTE LTD
PCI (Penn Pharmaceutical Services Ltd.), Tredegar
Active Ingredients
Documents
Package Inserts
Vitrakvi PI.pdf
Approved: November 7, 2023
