Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SUSPENSION
**4.2 Posology and method of administration** Abraxane should only be administered under the supervision of a qualified oncologist in units specialised in the administration of cytotoxic agents. It should not be substituted for or with other paclitaxel formulations. Posology _Breast cancer_ The recommended dose of Abraxane is 260 mg/m2 administered intravenously over 30 minutes every 3 weeks. _Dose adjustments during treatment of breast cancer_ Patients who experience severe neutropenia (neutrophil count < 500 cells/mm3 for a week or longer) or severe sensory neuropathy during Abraxane therapy should have the dose reduced to 220 mg/m2 for subsequent courses. Following recurrence of severe neutropenia or severe sensory neuropathy, additional dose reduction should be made to 180 mg/m2. Abraxane should not be administered until neutrophil counts recover to > 1500 cells/mm3. For Grade 3 sensory neuropathy, withhold treatment until resolution to Grade 1 or 2, followed by a dose reduction for all subsequent courses. _Pancreatic adenocarcinoma_ The recommended dose of Abraxane in combination with gemcitabine is 125 mg/m2 administered intravenously over 30 minutes on Days 1, 8 and 15 of each 28-day cycle. The concurrent recommended dose of gemcitabine is 1000 mg/m2 administered intravenously over 30 minutes immediately after the completion of Abraxane administration on Days 1, 8 and 15 of each 28-day cycle. _Dose adjustments during treatment of pancreatic adenocarcinoma_ 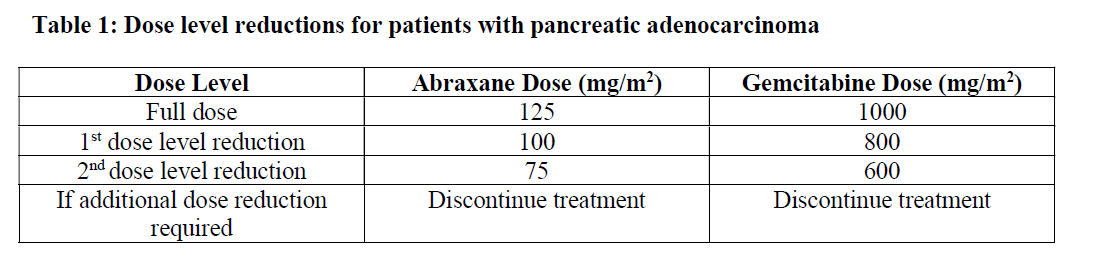 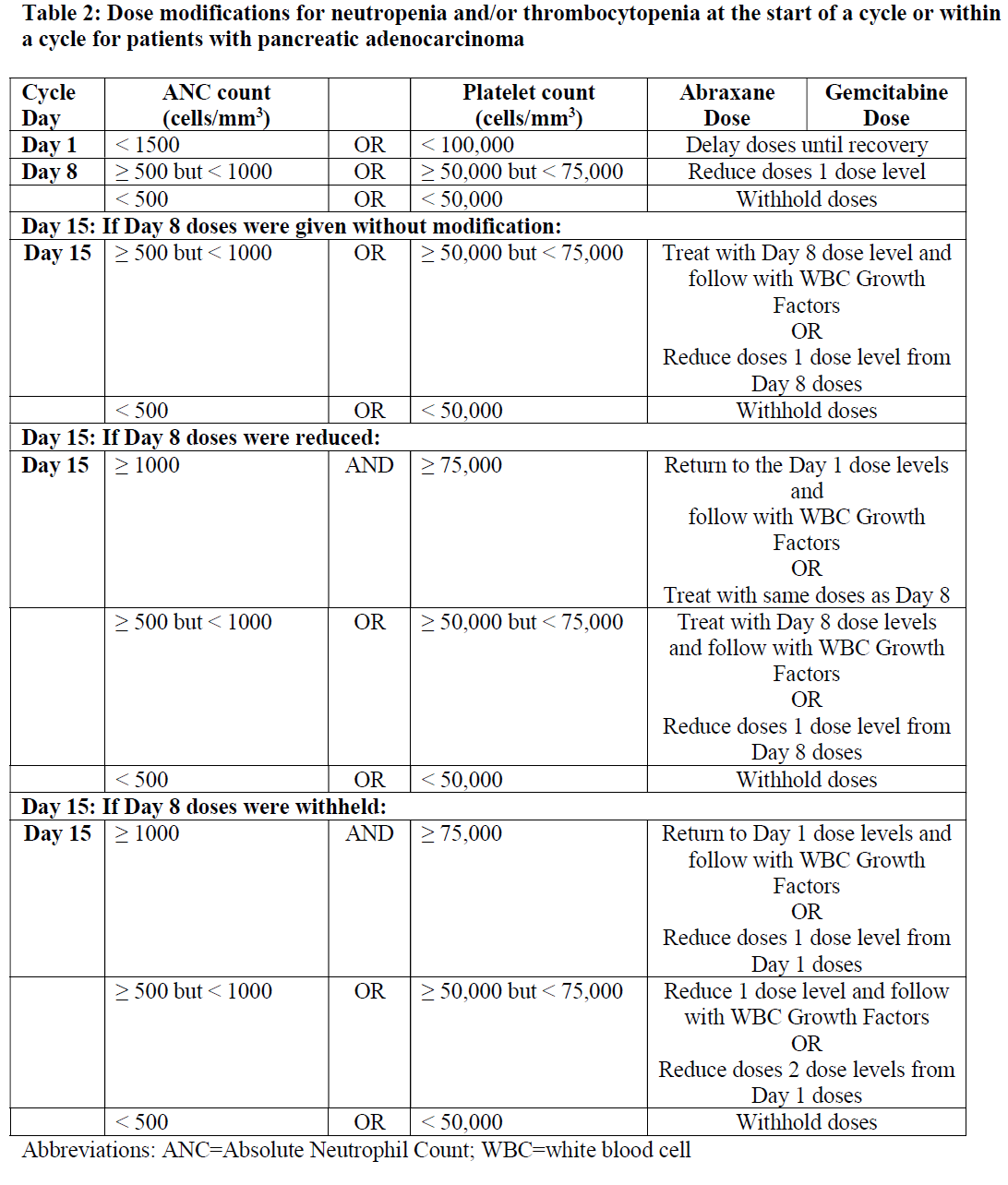 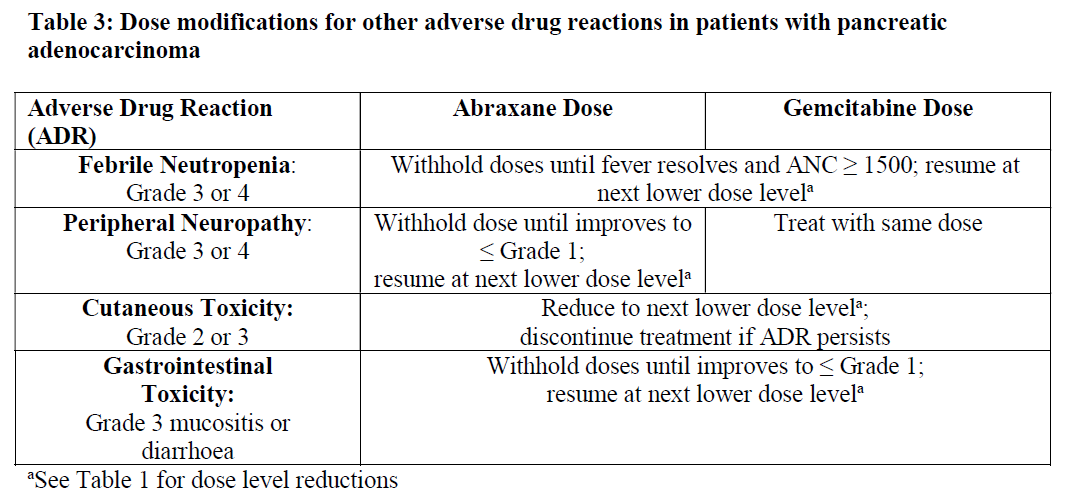 _Non-Small Cell Lung Cancer_ The recommended dose of ABRAXANE is 100 mg/m2 administered as an intravenous infusion over 30 minutes on Days 1, 8, and 15 of each 21-day cycle. The recommended dose of carboplatin is AUC = 6 mg•min/mL on Day 1 only of each 21-day cycle, beginning immediately after the end of Abraxane administration. _Dose adjustments during treatment of non-small cell lung cancer_ Do not administer ABRAXANE on Day 1 of a cycle until absolute neutrophil count (ANC) is at least 1500 cells/mm3 and platelet count is at least 100,000 cells/mm3. In patients who develop severe neutropenia or thrombocytopenia, withhold treatment until counts recover to an absolute neutrophil count of at least 1500 cells/mm3 and platelet count of at least 100,000 cells/mm3 on Day 1 or to an absolute neutrophil count of at least 500 cells/mm3 and platelet count of at least 50,000 cells/mm3 on Days 8 or 15 of the cycle. Upon resumption of dosing, permanently reduce ABRAXANE and carboplatin doses as outlined in Table 4. Withhold ABRAXANE for Grade 3–4 peripheral neuropathy. Resume ABRAXANE and carboplatin at reduced doses (see Table 4) when peripheral neuropathy improves to Grade 1 or completely resolves. 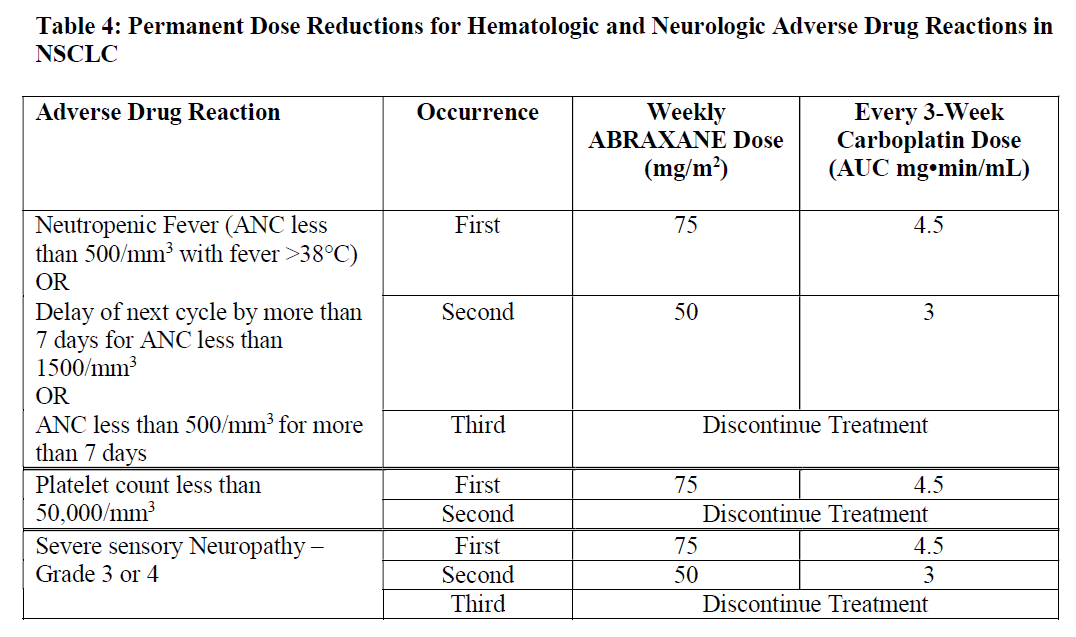 Special populations _Hepatic impairment_ For patients with mild hepatic impairment (total bilirubin > 1 to ≤ 1.5 x ULN and aspartate aminotransferase \[AST\] ≤ 10 x ULN), no dose adjustments are required, regardless of indication. Treat with same doses as patients with normal hepatic function. For metastatic breast cancer patients and non-small cell lung cancer patients with moderate to severe hepatic impairment (total bilirubin > 1.5 to ≤ 5 x ULN and AST ≤ 10 x ULN), a 20% reduction in dose is recommended. The reduced dose may be escalated to the dose for patients with normal hepatic function if the patient is tolerating the treatment for at least two cycles (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with metastatic adenocarcinoma of the pancreas that have moderate to severe hepatic impairment, there are insufficient data to permit dosage recommendations (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with total bilirubin > 5 x ULN or AST > 10 x ULN, there are insufficient data to permit dosage recommendations regardless of indication (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ Adjustment of the starting Abraxane dose is not required for patients with mild to moderate renal impairment (estimated creatinine clearance ≥30 to <90 ml/min). There are insufficient data available to recommend dose modifications of Abraxane in patients with severe renal impairment or end stage renal disease (estimated creatinine clearance <30 ml/min) (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Elderly_ No additional dosage reductions, other than those for all patients, are recommended for patients 65 years and older. Of the 229 patients in the randomized study who received Abraxane monotherapy for breast cancer, 13% were at least 65 years of age and < 2% were 75 years and older. No toxicities occurred notably more frequently among patients at least 65 years of age who received Abraxane. However, a subsequent analysis in 981 patients receiving Abraxane monotherapy for metastatic breast cancer, of which 15% were ≥ 65 years old and 2% were ≥ 75 years old, showed a higher incidence of epistaxis, diarrhoea, dehydration, fatigue and peripheral oedema in patients ≥ 65 years. Of the 421 patients with pancreatic adenocarcinoma in the randomized study who received Abraxane in combination with gemcitabine, 41% were 65 years and older and 10% were 75 years and older. In patients aged 75 years and older who received Abraxane and gemcitabine, there was a higher incidence of serious adverse reactions and adverse reactions that led to treatment discontinuation (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with pancreatic adenocarcinoma aged 75 years and older should be carefully assessed before treatment is considered (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Of the 514 patients in the randomized study who received ABRAXANE and carboplatin for the first-line treatment of non-small cell lung cancer, 31% were 65 years or older and 3.5% were 75 years or older. Myelosuppression, peripheral neuropathy, and arthralgia were more frequent in patients 65 years or older compared to patients younger than 65 years old. No overall difference in effectiveness, as measured by response rates, was observed between patients 65 years or older compared to patients younger than 65 years old. Pharmacokinetic/pharmacodynamic modelling using data from 125 patients with advanced solid tumours indicates that patients ≥ 65 years of age may be more susceptible to development of neutropenia within the first treatment cycle. _Paediatric population_ The safety and efficacy of Abraxane in children and adolescents aged 0 to less than 18 years has not been established. Currently available data are described in sections 4.8, 5.1 and 5.2 but no recommendation on a posology can be made – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. There is no relevant use of Abraxane in the paediatric population for the indication of metastatic breast cancer or pancreatic adenocarcinoma or non-small cell lung cancer. Method of administration Administer reconstituted Abraxane suspension intravenously using an infusion set incorporating a 15 micrometres filter. Following administration, it is recommended that the intravenous line be flushed with sodium chloride 9 mg/ml (0.9%) solution for injection to ensure administration of the complete dose. For instructions on reconstitution of the medicinal product before administration, see section 6.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS DRIP
Medical Information
**4.1 Therapeutic indications** Abraxane monotherapy is indicated for the treatment of metastatic breast cancer in adult patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline containing therapy is not indicated (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Abraxane in combination with gemcitabine is indicated for the first-line treatment of adult patients with metastatic adenocarcinoma of the pancreas. Abraxane in combination with carboplatin is indicated as first-line treatment of locally advanced or metastatic non-small cell lung cancer, in patients who are not candidates for curative surgery or radiation therapy.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Lactation (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients who have baseline neutrophil counts < 1500 cells/mm3.
L01CD01
paclitaxel
Manufacturer Information
CELGENE PTE. LTD.
Abraxis BioScience, LLC
Baxter Oncology GmbH
