Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED, EXTENDED RELEASE
**DOSAGE AND ADMINISTRATION** Oral use. For adult use only. _**DIAMICRON MR 30mg**_ tablets do not have a break bar and must not be broken in half. Tablets must be administered whole. _**DIAMICRON MR 60mg**_ tablets have a break bar and may be administered as whole or as half tablets (see Pharmacokinetics – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). So that the modified release properties of the product can be maintained, _**DIAMICRON MR 30mg**_ and _**DIAMICRON MR 60mg**_ tablets should not be chewed or crushed. These products should be taken with food because there is an increased risk of hypoglycemia if a meal is taken late, if an inadequate amount of food is consumed or if the food is low in carbohydrate. It is recommended that the medication be taken at breakfast time. If a dose is forgotten, the dose taken on the next day should not be increased. - A single daily dose provides an effective blood glucose control. The initial recommended dose for these products is 30mg daily, even in elderly patients (≥ 65 years). For _**DIAMICRON MR 30mg**_ the single daily dose may be between one (30mg) and four (120mg) tablets. - For _**DIAMICRON MR 60mg**_ the single daily dose may be between half a tablet (30mg) and two tablets (120mg). For both _**DIAMICRON MR 30mg**_ and _**DIAMICRON MR 60mg**_, the daily dose should not exceed 120mg. Previously untreated patients should commence with a dose of 30mg and will benefit from dose titration until the appropriate dose is reached. As with all hypoglycaemic agents, doses should be titrated according to the individual patient's response. Titration should be carried out in steps of 30mg, according to the fasting blood glucose response. Each step should last for at least one month between each increment, except in patients whose blood glucose do not decrease after two weeks of treatment. In this case, it is possible to propose a dosage increase at the end of the 2nd week treatment. Across the dose range, one half of a _**DIAMICRON MR 60mg**_ tablet, can replace one tablet of _**DIAMICRON MR 30mg**_, or one tablet of gliclazide 80mg immediate release (refer to the following table for other dose equivalences). 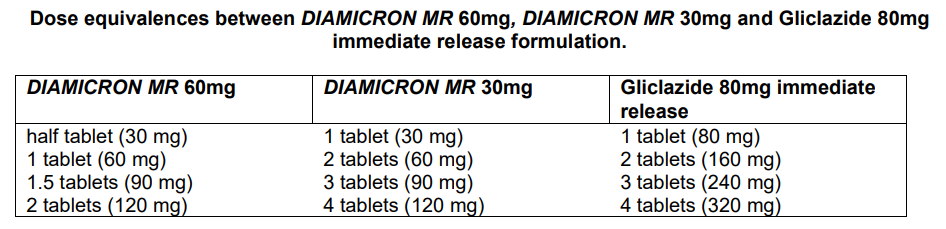 _**DIAMICRON MR 30mg**_ or _**DIAMICRON MR 60mg**_, may be used to replace other antidiabetic treatments without any transitional period. If a patient is switched from a hypoglycaemic sulfonylurea with a prolonged half-life he/she should be carefully monitored (for one to two weeks) in order to avoid hypoglycaemia due to possible residual effects of the previous therapy. _**DIAMICRON MR 30mg**_ or _**DIAMICRON MR 60mg**_, may be given in combination with biguanides, alpha glucosidase inhibitors or insulin. In patients not adequately controlled with _**DIAMICRON MR**_ concomitant insulin therapy can be initiated under close medical supervision. Elderly subjects: The efficacy and tolerance of the modified release formulation of gliclazide (30mg – 120mg) has been confirmed in clinical trials in subjects over 65 years who were given the same dosage regimen as the general population. The dosage is therefore identical to that recommended for adults under the age of 65 years. Renal insufficiency: The efficacy and tolerance of the modified release formulation of gliclazide (30mg – 120mg) has been confirmed in clinical trials of subjects with mild to moderate renal failure (creatinine clearance of between 15 and 80mL/min) who were given the same dosage regimen as the general population. No dosage adjustment is therefore required in subjects with impaired renal function. In patients at risk of hypoglycaemia: - states of undernourishment or malnutrition, - severe or poorly compensated endocrine pathologies (hypopituitarism, hypothyroidism, adrenal insufficiency), - withdrawal from prolonged and /or high dose corticosteroid therapy, - severe vascular disease (severe coronary heart disease, severe carotid impairment, diffuse vascular disease) It is recommended that treatment be systematically initiated with a minimal dose of 30mg / day. There are no data or clinical studies in children.
ORAL
Medical Information
**INDICATIONS** _**DIAMICRON MR 30mg**_ and _**DIAMICRON MR 60mg**_ are indicated for the treatment of type II diabetes in association with dietary measures and with, physical exercise when these measures alone are inadequate to control blood glucose. During controlled clinical trials in patients with type II diabetes, a modified release formulation of gliclazide (30mg – 120mg), taken as a single daily dose, was shown to be effective long term in controlling blood glucose levels, based on monitoring of HbA1c.
**CONTRAINDICATIONS** _**DIAMICRON MR 30mg**_ and _**DIAMICRON MR 60mg**_ are contra-indicated in the following cases: - hypersensitivity to gliclazide, other sulfonylureas, sulphonamides, or to any of the excipients; - type 1 diabetes; - diabetic pre-coma and coma; diabetic keto-acidosis; - severe renal or hepatic insufficiency: in these cases, the use of insulin is recommended; - treatment with miconazole (refer to Interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_); - pregnancy and lactation (refer to Use in pregnancy and Use in lactation – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
A10BB09
gliclazide
Manufacturer Information
SERVIER (S) PTE LTD
LES LABORATOIRES SERVIER INDUSTRIE
SERVIER (IRELAND) INDUSTRIES LTD
Kotra Pharma (M) Sdn Bhd
Active Ingredients
Documents
Package Inserts
Diamicron MR Tablet PI.pdf
Approved: May 27, 2020
