Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**Posology and method of administration** - 75 mg doses can be administered as one 75 mg capsule. - dosage of 30mg and 45mg cannot be administered using the 75mg capsules. _Treatment of influenza_ Treatment should be initiated as soon as possible within the first two days of onset of symptoms of influenza. For adolescents (13 to 17 years of age) and adults: The recommended oral dose is 75 mg oseltamivir twice daily for 5 days. For infants older than 1 year of age and for children 2 to 12 years of age: The recommended dose of Oseltamivir Phosphate is indicated in the table below. The following weight-adjusted dosing regimens are recommended 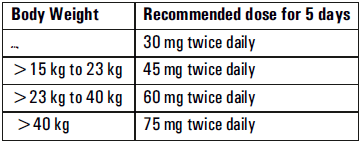 For children 6 to 12 months of age: Depending on the pathogenicity of the circulating influenza virus strain, children between 6 and 12 months of age can be treated with Oseltamivir Phosphate during a pandemic influenza outbreak, although the available data are limited. Pharmacokinetic data indicate that a dosage of 3 mg/kg twice daily in children 6 to 12 months of age provides plasma drug exposures in the majority of patients similar to those shown to be clinically efficacious in children age one or older and adults (see section Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended dose for treatment of children 6 to 12 months of age is 3 mg per kg body weight twice daily for 5 days for treatment. _Prevention of influenza_ _Post-exposure prevention_ For adolescents (13 to 17 years of age) and adults: The recommended dose for prevention of influenza following close contact with an infected individual is 75 mg oseltamivir once daily for 10 days. Therapy should begin as soon as possible within two days of exposure to an infected individual. For infants older than 1 year of age and for children 2 to 12 years of age: The recommended post-exposure prevention dose of Oseltamivir Phosphate is: 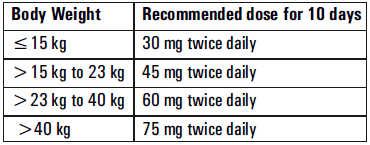 _Prevention during an influenza epidemic in the community_ The recommended dose for prevention of influenza during a community outbreak is 75 mg oseltamivir once daily for up to 6 weeks. **Special populations** _Hepatic impairment_ No dose adjustment is required either for treatment or for prevention in patients with hepatic dysfunction. No studies have been carried out in paediatric patients with hepatic disorder. _Renal impairment_ _Treatment of influenza_: Dose adjustment is recommended for adults and adolescents (13 to 17 years of age) with moderate or severe renal impairment. Recommended doses are detailed in the table below. 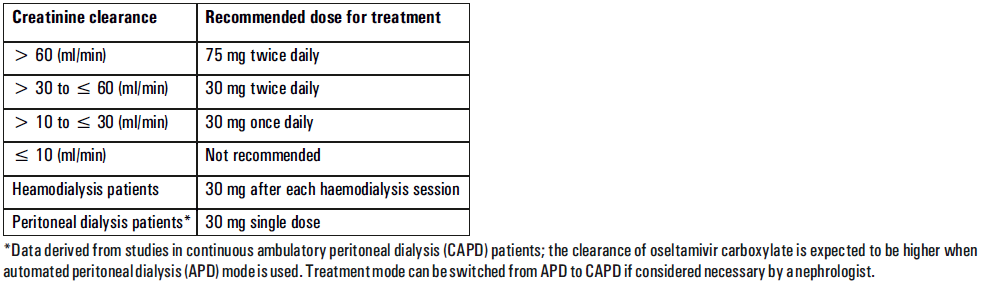 _Prevention of influenza_: Dose adjustment is recommended for adults and adolescents (13 to 17 years of age) with moderate or severe renal impairment as detailed in the table below. 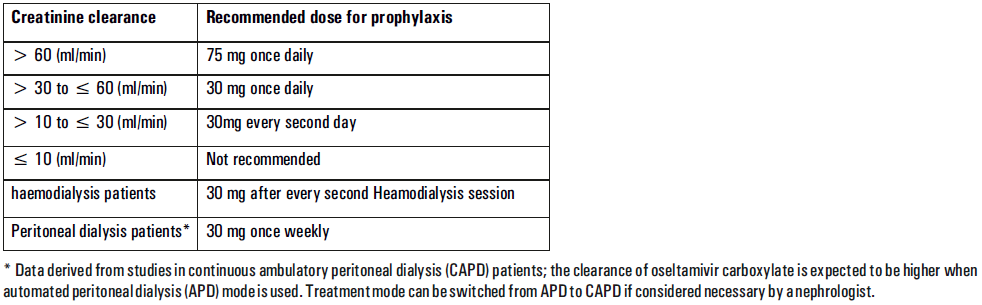 _Geriatric Use_ No dose adjustment is required, unless there is evidence of severe renal impairment. _Children_ There is insufficient clinical data available in children with renal impairment to be able to make any dosing recommendation.
ORAL
Medical Information
**Therapeutic indications** _Treatment of influenza_ In patients one year of age and older who present with symptoms typical of influenza, when influenza virus is circulating in the community. Efficacy has been demonstrated when treatment is initiated within two days of first onset of symptoms. This indication is based on clinical studies of naturally occurring influenza in which the predominant infection was influenza A (see section Pharmacodynamic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Based on limited pharmacokinetic and safety data, Oseltamivir Phosphate can be used in children 6 to 12 months of age for treatment during a pandemic influenza outbreak. The treating physician should take into account the pathogenicity of the circulating strain and the underlying condition of the patient to ensure there is a potential benefit to the child. _Prevention of influenza_ - Post-exposure prevention in individuals one year of age or older following contact with a clinically diagnosed influenza case when influenza virus is circulating in the community. - The appropriate use of Oseltamivir Phosphate for prevention of influenza should be determined on a case by case basis by the circumstances and the population requiring protection. In exceptional situations (e.g., in case of a mismatch between the circulating and vaccine virus strains, and a pandemic situation) seasonal prevention could be considered in individuals one year of age or older. Oseltamivir Phosphate is not a substitute for influenza vaccination The use of antivirals for the treatment and prevention of influenza should be determined on the basis of official recommendations. Decisions regarding the use of antivirals for treatment and prophylaxis should take into consideration what is known about the characteristics of the circulating influenza viruses and the impact of the disease in different geographical areas and patient populations.
**Contraindications** Oseltamivir Phosphate is contraindicated in patients with known hypersensitivity to Oseltamivir phosphate or to any component of the product.
J05AH02
oseltamivir
Manufacturer Information
HETERO SINGAPORE PTE. LTD.
Hetero labs limited
Active Ingredients
Documents
Package Inserts
1.4.3 Package Insert (PI).pdf
Approved: August 24, 2021
