Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2.2. Dosage and Administration** **Standard Dosage** Xeloda should only be prescribed by a qualified physician experienced in the utilisation of anti-neoplastic agents. Xeloda tablets should be swallowed whole with water within 30 minutes after a meal. Xeloda tablets should not be crushed or cut. If patients cannot swallow Xeloda tablets whole and tablets must be crushed or cut, this should be done by a professional trained in the safe handling of cytotoxic drugs (see section 4.2 Special Instructions for Use, Handling and Disposal – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Treatment should be discontinued if progressive disease or intolerable toxicity is observed. Standard and reduced dose calculations according to body surface area for starting doses of Xeloda of 1250 mg/m2 and 1000 mg/m2 are provided in tables 1 and 2, respectively. Monotherapy _Colon, colorectal and breast cancer:_ The recommended monotherapy starting dose of Xeloda in the adjuvant treatment of colon cancer, in the treatment of metastatic colorectal cancer or of locally advanced or metastatic breast cancer is 1250 mg/m2 administered twice daily (morning and evening; equivalent to 2500 mg/m2 total daily dose) for 2 weeks followed by a 7-day rest period. Adjuvant treatment in patients with stage III colon cancer is recommended for a total of 6 months. Combination therapy _Breast cancer:_ In combination with docetaxel, the recommended starting dose of Xeloda is 1250 mg/m2 twice daily for 2 weeks followed by a 7-day rest period, combined with docetaxel at 75 mg/m2 as a 1-hour intravenous infusion every 3 weeks. Pre-medication with an oral corticosteroid such as dexamethasone according to the docetaxel summary of product characteristics should be started prior to docetaxel administration for patients receiving the Xeloda plus docetaxel combination. _Colon, colorectal and gastric cancer:_ In combination treatment, the recommended starting dose of Xeloda should be reduced to 1000 mg/m2 administered twice daily for 2 weeks followed by a 7-day rest period. (see section 3.1.2 Clinical/ Efficacy studies for further information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The inclusion of bevacizumab in a combination regimen has no effect on the starting dose of Xeloda. Premedication to maintain adequate hydration and anti-emesis according to the cisplatin and oxaliplatin product information should be started prior to cisplatin administration for patients receiving the Xeloda plus cisplatin or oxaliplatin combination. Adjuvant treatment in patients with stage III colon cancer is recommended for a total of 6 months. Dose calculation Xeloda dose is calculated according to body surface area. The following tables show examples of the standard and reduced dose calculations (see section “Dosage adjustments during treatment”) for a starting dose of Xeloda of either 1250 mg/m2 or 1000 mg/m2. 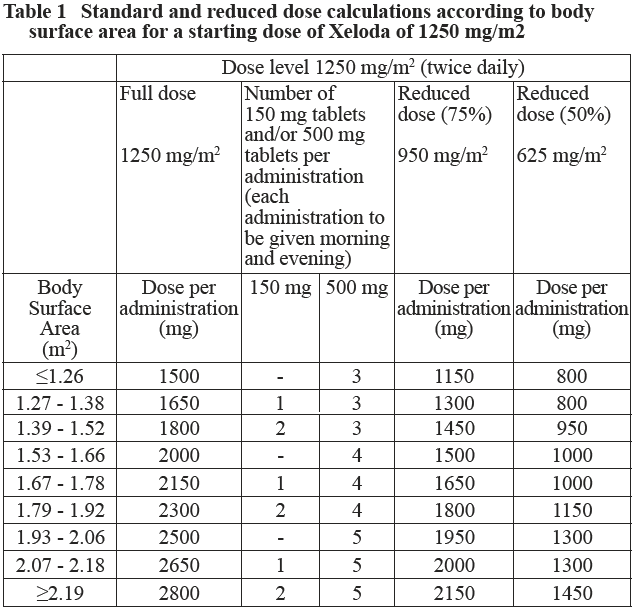 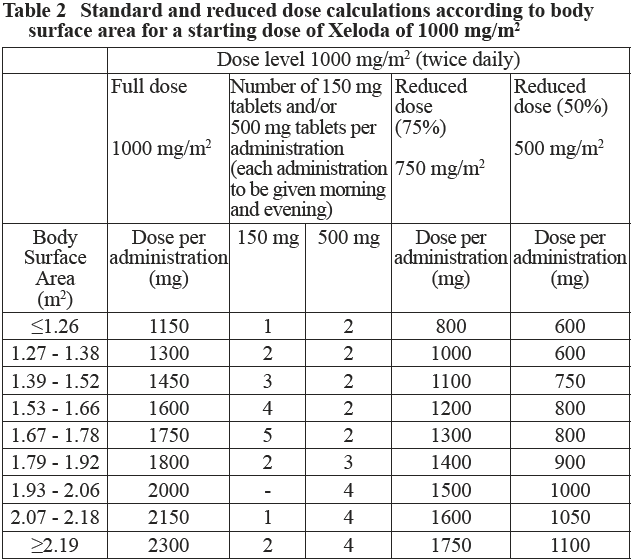 Dosage adjustments during treatment _General:_ Toxicity due to Xeloda administration may be managed by symptomatic treatment and/or modification of the Xeloda dose (treatment interruption or dose reduction). Once the dose has been reduced it should not be increased at a later time. For those toxicities considered by the treating physician to be unlikely to become serious or life-threatening treatment can be continued at the same dose without reduction or interruption. Patients taking Xeloda should be informed of the need to interrupt treatment immediately if moderate or severe toxicity occurs. Doses of Xeloda omitted for toxicity are not replaced. _Hematology:_ Patients with baseline neutrophil counts of <1.5 x 109 /l and/or thrombocyte counts of <100 x 109 /l should not be treated with Xeloda. If unscheduled laboratory assessments during a treatment cycle show grade 3 or 4 hematologic toxicity, treatment with Xeloda should be interrupted. If the neutrophil count drops below 1.0 x 109/L or if the platelet count drops below 75 x 109/L, capecitabine. At recovery, restart capecitabine at full dose. The following table shows the recommended dose modifications following toxicity related to Xeloda: 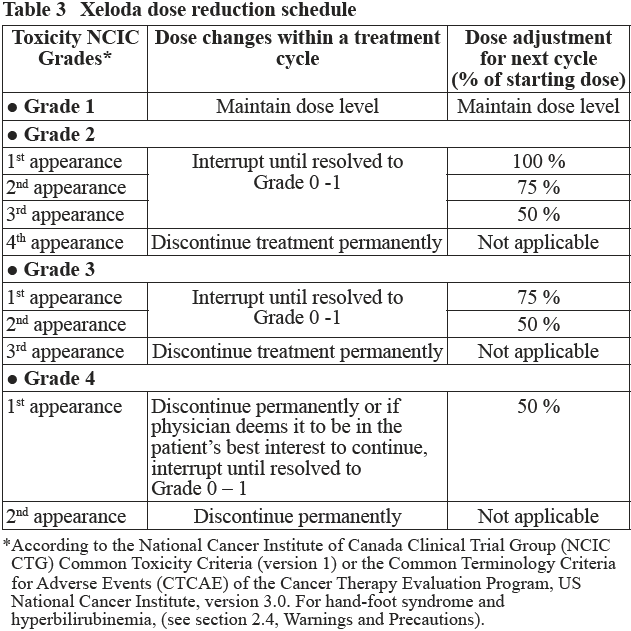 General combination therapy Dose modifications for toxicity when Xeloda is used in combination with other therapies should be made according to Table 3 above for Xeloda and according to the appropriate Prescribing Information for the other agent(s). At the beginning of a treatment cycle, if a treatment delay is indicated for either Xeloda or the other agent(s), then administration of all agents should be delayed until the requirements for restarting all drugs are met. During a treatment cycle for those toxicities considered by the treating physician not to be related to Xeloda, Xeloda should be continued and the dose of the other agent adjusted according to the appropriate Prescribing Information. If the other agent(s) have to be discontinued permanently, Xeloda treatment can be resumed when the requirements for restarting Xeloda are met. This advice is applicable to all indications and to all special populations. **2.2.1. Special Dosage Instructions** _Pediatric use_ The safety and efficacy of Xeloda in children and adolescents (< 18 years) have not been established. _Geriatric use_ For Xeloda monotherapy, no adjustment of the starting dose is needed. However, severe grade 3 or 4 treatment-related adverse drug reactions (ADRs) were more frequent in patients over 80 years of age compared to younger patients. When Xeloda was used in combination with other antineoplastic agents, geriatric patients (≥ 65 years) experienced more grade 3 and grade 4 ADRs and ADRs that led to discontinuation, than younger patients. Careful monitoring of elderly patients is advisable. In combination with docetaxel: an increased incidence of grade 3 or 4 treatment-related ADRs and treatment-related serious ADRs was observed in patients 60 years of age or more. For patients 60 years of age or more treated with the combination of Xeloda plus docetaxel, a starting dose reduction of Xeloda to 75% (950 mg/m2 twice daily) is recommended. For dosage calculations, see Table 2. In combination with irinotecan: for patients 65 years of age or more, a starting dose reduction of Xeloda to 800 mg/m2 twice daily is recommended. _Renal Impairment_ In patients with moderate renal impairment (creatinine clearance 30 – 50 ml/min \[Cockroft and Gault\]) at baseline, a dose reduction to 75% for a starting dose of 1250 mg/m2 is recommended. In patients with mild renal impairment (creatinine clearance 51 – 80 ml/min), no adjustment in starting dose is recommended. Careful monitoring and prompt treatment interruption is recommended if the patient develops a Grade 2, 3, or 4 ADRs with subsequent dose adjustment as outlined in Table 3 above (see also section 3.2.5 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If the calculated creatinine clearance decreases during treatment to a value below 30 ml/min, Xeloda should be discontinued. The dose adjustment recommendations for patients with moderate renal impairment apply both to monotherapy and combination use. For dosage calculations, see Tables 1 and 2. _Hepatic Impairment_ In patients with mild to moderate hepatic impairment due to liver metastases, no starting dose adjustment is necessary. However, such patients should be carefully monitored (see section 3.2.5, Pharmacokinetics in Special Populations and section 2.4, Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with severe hepatic impairment have not been studied.
ORAL
Medical Information
**2.1. Therapeutic Indication(s)** _Breast Cancer:_ Xeloda in combination with docetaxel is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline. Xeloda is also indicated as monotherapy for the treatment of patients with locally advanced or metastatic breast cancer after failure of a taxane and an anthracycline-containing chemotherapy regimen or for whom further anthracycline therapy is not indicated. _Colorectal cancer:_ Xeloda is indicated for the adjuvant treatment of patients following surgery of stage III (Dukes' stage C) colon cancer. Xeloda is indicated for the treatment of metastatic colorectal carcinoma. _Gastric Cancer_ Xeloda is indicated for first-line treatment of advanced gastric cancer in combination with a platinum-based regimen.
**2.3. Contraindications** Xeloda is contraindicated in patients with a known hypersensitivity to capecitabine or to any of its excipients. Xeloda is contraindicated in patients who have a history of severe and unexpected reactions to fluoropyrimidine therapy or with known hypersensitivity to fluorouracil. As with other fluoropyrimidines, Xeloda is contraindicated in patients with known complete absence of dihydropyrimidine dehydrogenase DPD activity (see section 2.4 Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Xeloda should not be administered concomitantly with sorivudine or its chemically related analogues, such as brivudine (see section 2.4.2 Interactions with other Medicinal Products and other Forms of Interaction – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Xeloda is contraindicated in patients with severe leukopenia, neutropenia or thrombocytopenia. Xeloda is contraindicated in patients with severe hepatic impairment. Xeloda is contraindicated in patients with severe renal impairment (creatinine clearance below 30 ml/min). If contraindications exist to any of the agents in a combination regimen, that agent should not be used.
L01BC06
capecitabine
Manufacturer Information
DKSH SINGAPORE PTE. LTD.
F HOFFMANN-LA ROCHE LTD (Primary and Secondary Packager)
Excella GmbH & Co. KG
Active Ingredients
Documents
Package Inserts
XELODA TABLETS PI.pdf
Approved: December 2, 2022
