Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION, CONCENTRATE
**POSOLOGY AND METHOD OF ADMINISTRATION:** **All patients must be premedicated prior to CYTAX administration to reduce the risk of severe hypersensitivity reactions. Such premedication may consist of dexamethasone 20 mg orally (or its equivalent) approximately 12 and 6 hours before CYTAX or 20mg I.V. approximately 30 to 60 minutes before CYTAX, diphenhydramine 50 mg I.V. (or its equivalent) 30 to 60 minutes prior to CYTAX and cimetidine (300 mg) or ranitidine (50 mg) I.V. 30 to 60 minutes prior to CYTAX.** Repeat courses of CYTAX should not be administered to patients with solid tumours until the neutrophil count is at least 1500 cells/mm3 and the platelet count is at least 100,000 cells/mm3 (<1000 cells/mm3 for patients with Kaposi’s sarcoma). Patients who experience severe neutropenia (<500 cells/mm3) or severe peripheral neuropathy should receive a dosage reduced by 20% for subsequent courses. The incidence of neurotoxicity and the severity of neutropenia increase with dose within a regimen. **Metastatic Carcinoma of the Ovary:** _Combination therapy:_ For previously untreated patients, the recommended dosing regimen, given every 3 weeks, is CYTAX administered intravenously over 3 hours at a dose of 175 mg/m2 followed by a platinum compound. Alternatively, a more myelosuppressive regimen of CYTAX may also be administered intravenously at a dose of 135 mg/m2 over 24 hours followed by a platinum compound, every 3 weeks. _Single-agent therapy:_ In patients previously treated with chemotherapy the recommended regimen is 175 mg/m2 administered intravenously over 3 hours every 3 weeks. **Carcinoma of the Breast:** _Adjuvant therapy:_ CYTAX 175 mg/m2 administered intravenously over 3 hours every 3 weeks for 4 courses sequentially to standard combination therapy. _Single-agent, first-line therapy after relapse within 6 months of adjuvant therapy:_ CYTAX 175 mg/m2 administered intravenously over 3 hours every 3 weeks. _Combination, first-line therapy of advanced or metastatic breast cancer:_ In combination with trastuzumab, the recommended dose of CYTAX is 175 mg/m2 administered intravenously over a period of 3 hours, with a 3-week interval between courses. CYTAX infusion may be started the day following the first dose of trastuzumab or immediately after the subsequent doses of trastuzumab if the preceding dose of trastuzumab was well tolerated. _Combination, first-line therapy of metastatic breast cancer:_ In combination with doxorubicin (50 mg/m2), CYTAX should be administered 24 hours after doxorubicin. The recommended dose of CYTAX is 220 mg/m2 administered intravenously over a period of 3 hours, with a 3-week interval between courses. _Single-agent second-line therapy after failure of combination chemotherapy for metastatic disease:_ CYTAX 175 mg/m2 administered intravenously over 3 hours every 3 weeks. **Non-Small Cell Lung Carcinoma:** _Combination therapy:_ For previously untreated patients, the recommended dosing regimen given with a 3-week interval between courses is, CYTAX 175 mg/m2 administered intravenously over 3 hours followed by a platinum compound. Alternatively, a more myelosuppressive regimen of CYTAX may be administered intravenously, 135 mg/m2 over 24 hours followed by a platinum compound, with a 3-week interval between courses. _Single-agent therapy:_ CYTAX 175 to 225 mg/m2 administered intravenously over 3 hours every 3 weeks. **AIDS-Related Kaposi’s Sarcoma:** _Second-line therapy:_ CYTAX 135 mg/m2 administered intravenously over 3 hours with a 3-week interval between courses or 100mg/m2 administered intravenously over 3 hours with a 2-week interval between courses (dose intensity 45–50 mg/m2/week). Based upon the immunosuppression observed in patients with advanced HIV disease, the following modifications are recommended in these patients. 1. the dose of dexamethasone as one of the three premedication drugs should be reduced to 10 mg orally 2. treatment with CYTAX should be initiated or repeated only if the neutrophil count is at least 1000 cells/mm3 3. the dose of subsequent courses of CYTAX should be reduced by 20% for those patients who experience severe neutropenia (<500 cells/mm3 for a week or longer) 4. concomitant hematopoietic growth factor (G-CSF), should be initiated as clinically indicated. _Hepatic Impairment_ Patients with hepatic impairment may be at increased risk of toxicity, particularly grade III–IV myelosuppression. Dose adjustment is recommended, as shown in Table 1 for both 3- and 24-hour infusions. Patients should be monitored closely for the development of profound myelosuppression. 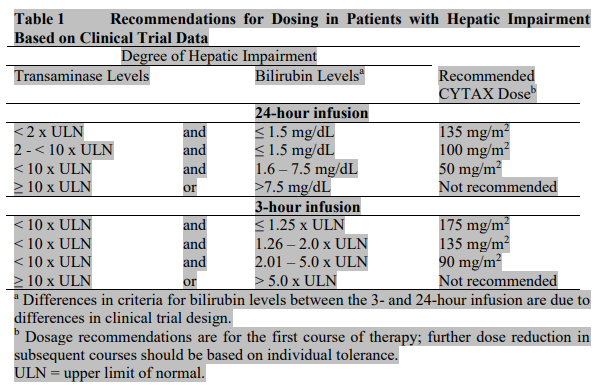 _Incompatibilities_ Contact of the undiluted concentrate with plasticized PVC equipment or devices used to prepare solutions for infusion is not recommended. In order to minimize patient exposure to the plasticizer DEHP \[di-(2-ethylhexyl)phthalate\], which may be leached from PVC infusion bags or sets, diluted TAXOL solutions should be stored in bottles (glass, polypropylene) or plastic bags (polypropylene, polyolefin) and administered through polyethylene-lined administration sets. **Mode of Administration:** CYTAX® Injection USP Concentrate 6mg/ml is for IV use.
INTRAVENOUS
Medical Information
**INDICATIONS:** _Ovarian Carcinoma_ - First-line therapy in combination with a platinum compound for the treatment of advanced metastatic carcinoma of the ovary. - Second-line therapy for the treatment of advanced metastatic carcinoma of the ovary. _Breast Carcinoma_ - Adjuvant treatment of node-positive breast cancer administered sequentially to standard combination therapy. - First-line therapy of advanced or metastatic breast cancer after relapse within 6 months of adjuvant therapy. Prior therapy should have included an anthracycline unless clinically contraindicated. - First-line therapy of metastatic breast cancer in combination with trastuzumab in patients who over-express HER-2 as determined by immunohistochemistry. - First-line therapy of metastatic breast cancer in combination with an anthracycline in patients for whom anthracycline therapy is suitable. - Second-line therapy of advanced or metastatic breast cancer after failure of combination chemotherapy for metastatic disease. Prior therapy should have included an anthracycline unless clinically contraindicated. _Non-Small Cell Lung Carcinoma_ - First-line therapy in combination with a platinum compound or as a single agent for the treatment of non-small cell carcinoma of the lung in patients who are not candidates for potentially curative surgery and/or radiation therapy. _Kaposi’s Sarcoma_ - Second-line treatment of AIDS-related Kaposi’s Sarcoma
**Contraindications:** Paclitaxel is contraindicated in patients with severe hypersensitivity reactions to Paclitaxel, macroglycerol ricinoleate (polyoxyl castor oil) or to any of the excipients. Paclitaxel is contraindicated during pregnancy and lactation. Paclitaxel should not be used in patients with baseline neutrophils < 1.5 x 109/l (<1 x 109/l for KS patients) or platelets < 100 x 109/l (<75 x 109/l for KS patients). In KS, paclitaxel is also contraindicated in patients with concurrent, serious, uncontrolled infections. Patients with severe hepatic impairment must not be treated with Paclitaxel.
L01C D01
xl 01 c d 01
Manufacturer Information
ACCORD HEALTHCARE PRIVATE LIMITED
INTAS PHARMACEUTICALS LIMITED
Active Ingredients
Documents
Package Inserts
Cytax _PI.pdf
Approved: February 19, 2019
