Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**2 DOSAGE AND ADMINISTRATION** **For infiltration and intramuscular use only.** **The strength of HYPERRAB is 300 international units/mL.** **2.1 Dose** Use HYPERRAB in combination with rabies vaccine series to be effective. Do not use HYPERRAB alone for prevention. Administer HYPERRAB within 7 days after the first dose of rabies vaccine. 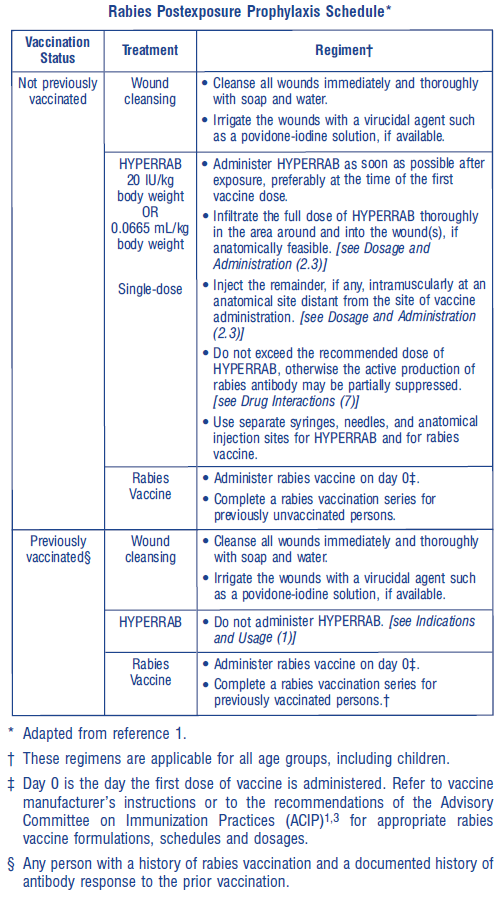 **2.2 Preparation** - Calculate the volume of HYPERRAB for the recommended dose of 20 international units/kg. - Ensure the correct strength is used for the calculation. HYPERRAB is formulated with a strength of 300 international units/mL. The predecessor product, HYPERRAB® S/D \[rabies immune globulin (human)\] was formulated at 150 international units/mL. The volume required of HYPERRAB (300 international units/mL) to achieve the recommended dose of 20 international units/kg is approximately one half of that required for the previous HYPERRAB S/D (150 international units/mL). - Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. HYPERRAB is a clear or slightly opalescent, and colorless or pale yellow or light brown sterile solution. - Do not use HYPERRAB if the product shows any sign of tampering. Notify Grifols Therapeutics LLC immediately \[1-800-520-2807\]. - Do not freeze. Do not use any solution that has been frozen. **2.3 Administration** - Administer HYPERRAB at the time of the first vaccine dose (day 0), but no later than day 7.1–3 - Infiltrate the full dose of HYPERRAB in the area around the wound, if anatomically feasible. Dilute HYPERRAB with an equal volume of dextrose, 5% (D5W), if additional volume is needed to infiltrate the entire wound. Do not dilute with normal saline. - Inject the remainder, if any, of the HYPERRAB dose intramuscularly into the deltoid muscle of the upper arm or into the lateral thigh muscle, and distant from the site of vaccine administration. - Do not administer HYPERRAB in the same syringe or needle or in the same anatomic site as vaccine. * * * **15 REFERENCES** 1. Centers for Disease Control and Prevention. Human rabies prevention – United States, 2008: Recommendations of the Advisory Committee on Immunization Practices. MMWR. 2008;57(RR03):1–26, 28. 2. World Health Organization. WHO Expert Consultation on Rabies: Second report. 2013. WHO technical report series, No. 982. 3. Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies. Recommendations of the Advisory Committee on Immunization Practices. MMWR. 2010;59(RR02):1–9. Erratum in: MMWR 2010;59(16):493.
INTRAMUSCULAR, INFILTRATION
Medical Information
**1 INDICATIONS AND USAGE** HYPERRAB is a human rabies immune globulin indicated for postexposure prophylaxis, along with rabies vaccine, for all persons suspected of exposure to rabies. Limitations of Use Persons who have been previously immunized with rabies vaccine and have a confirmed adequate rabies antibody titer should receive only vaccine.1–3 For unvaccinated persons, the combination of HYPERRAB and vaccine is recommended for both bite and nonbite exposures regardless of the time interval between exposure and initiation of postexposure prophylaxis.1–3 Beyond 7 days (after the first vaccine dose), HYPERRAB is not indicated since an antibody response to vaccine is presumed to have occurred. * * * **15 REFERENCES** 1. Centers for Disease Control and Prevention. Human rabies prevention – United States, 2008: Recommendations of the Advisory Committee on Immunization Practices. MMWR. 2008;57(RR03):1–26, 28. 2. World Health Organization. WHO Expert Consultation on Rabies: Second report. 2013. WHO technical report series, No. 982. 3. Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies. Recommendations of the Advisory Committee on Immunization Practices. MMWR. 2010;59(RR02):1–9. Erratum in: MMWR 2010;59(16):493.
**4 CONTRAINDICATIONS** None.
J06BB05
rabies immunoglobulin
Manufacturer Information
GRIFOLS ASIA PACIFIC PTE. LTD.
Grifols Therapeutics LLC
Active Ingredients
Documents
Package Inserts
HyperRAB Injection PI.pdf
Approved: June 14, 2023
