Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**DOSAGE AND ADMINISTRATION** As with all local anaesthetics, the dosage varies and depends upon the area to be anaesthetised, the vascularity of the tissues, the number of neuronal segments to be blocked, the depth of anaesthesia and degree of muscle relaxation required, individual tolerance, the technique of anaesthesia, and the physical condition of the patient. The lowest dosage that results in effective anaesthesia should be used. In general, surgical anaesthesia requires the use of higher concentrations and doses than those required for analgesia. The volume of the drug used will affect the extent of spread of anaesthesia. The presentations of MARCAIN injection solutions are intended for single use only. Any solution remaining from an opened container should be discarded. The following tables are a guide to dosage. The clinician’s experience and knowledge of the patient’s physical status are of importance in deciding the dose. Experience to date indicates that 400 mg administered over 24 hours is well tolerated in average adults. Dosage recommendations for MARCAIN for various anaesthetic procedures in an average, healthy 70 kg adult patient. **RECOMMENDED DOSAGE FOR SURGICAL ANAESTHESIA FOR MARCAIN** 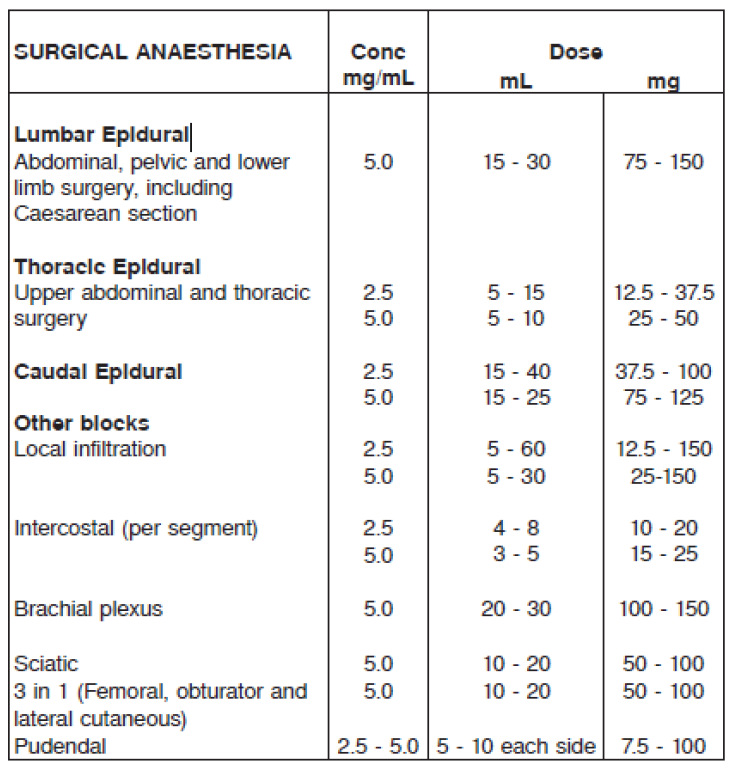 **RECOMMENDED DOSAGE FOR ANALGESIA, INCLUDING CONTINUOUS INFUSION** 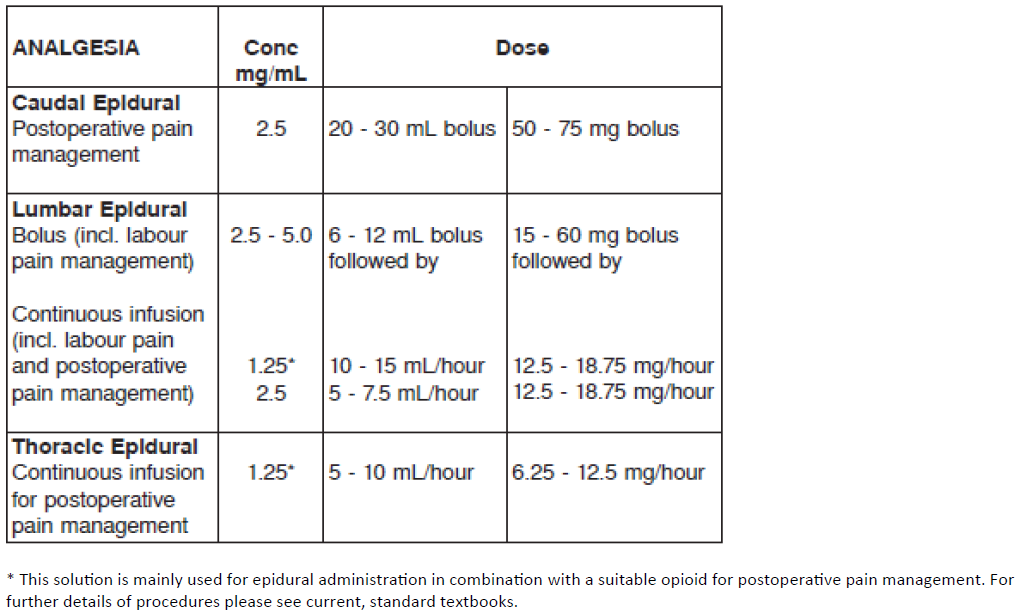 **NOTE**: 1. Recommended doses Tolerability varies widely between patients and toxic effects may occur after any local anaesthetic procedure. Careful observation of the patient must therefore be maintained. It is recommended that the dose of bupivacaine at any time should not exceed 2 mg/kg. However, the dose administered must be tailored to the individual patient and procedure, and the maximum dose quoted here should be used as a guide only. 2. Injection Injection of repeated doses of bupivacaine may cause significant increase in blood levels with each repeated dose, due to accumulation of the drug or its metabolites, or due to slow metabolic degradation. The rapid injection of a large volume of local anaesthetic solution should be avoided and fractional doses should be used when feasible. For most indications the duration of MARCAIN is such that a single dose is sufficient. 3. Hypotension During thoracic, lumbar and caudal epidural anaesthesia/analgesia, a marked fall in blood pressure and/or intercostal paralysis may be seen, possibly due to the use of excessive doses, improper positioning of the patient or accidental disposition of the anaesthetic within the subarachnoid space. Hypotension and bradycardia may occur as a result of sympathetic blockade. 4. Test dose For epidural anaesthesia, a test dose of 3–5 mL of a local anaesthetic solution, preferably containing up to 15 micrograms of adrenaline, should be administered. Verbal contact and repeated monitoring of heart rate and blood pressure should be maintained for 5 minutes following the test dose after which, in the absence of signs of subarachnoid or intravascular injection, the main dose may be given. Use of a test dose containing adrenaline may have further advantages in that an intravascular injection of adrenaline will be quickly recognised by an increase in heart rate, usually within about 40 seconds. To detect this, the heart rate and rhythm should be monitored with an electrocardiogram. An accidental intrathecal injection may be recognised by signs of a spinal block. Prior to administration of the total dose, aspiration should be repeated. The main dose should be injected **slowly** at a rate of 25–50 mg/min, while closely observing the patient’s vital functions and maintaining verbal contact. If toxic symptoms or signs occur, the injection should be stopped immediately. 5. Prolonged blocks When prolonged blocks are used, either by continuous infusion or by repeated bolus administration, the risks of reaching a toxic plasma concentration or inducing a local neural injury must be considered. Use in Children Experience with bupivacaine in children under the age of 12 is limited. The dosage in children should be calculated on a weight basis up to 2 mg/kg. Paediatric regional anaesthetic procedures should be performed by qualified clinicians who are familiar with this population and the technique. Use in Pregnancy It should be noted that the dose should be reduced in patients in the late stages of pregnancy. Use in Debilitated or Elderly Patients Debilitated or elderly patients, including those with partial or complete heart block, advanced liver disease or severe renal dysfunction should be given a reduced dosage commensurate with their physical condition (see PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRATHECAL
Medical Information
**INDICATIONS** MARCAIN solutions are indicated for the production of local or regional anaesthesia and analgesia in individuals as follows: Surgical anaesthesia - Epidural block for surgery. - Field block (minor and major nerve blocks and infiltration). Analgesia - Continuous epidural infusion or intermittent bolus epidural administration for analgesia in postoperative pain or labour pain. - Field block (minor nerve block and infiltration).
**CONTRAINDICATIONS** 1. Allergy or hypersensitivity to amide type local anaesthetics. Detection of suspected hypersensitivity by skin testing is of limited value. 2. Epidural and spinal anaesthesia is contraindicated in patients with uncorrected hypotension. 3. Local anaesthetic techniques must not be used when there is infection in the region of the proposed injection and/or in the presence of septicaemia. 4. Bupivacaine is contraindicated in obstetric paracervical block, intravenous regional anaesthesia (Bier’s block) and all intravenous infusions. 5. General contraindications related to epidural anaesthesia, regardless of the local anaesthetic used, should be taken into account.
N01BB01
bupivacaine
Manufacturer Information
DCH AURIGA SINGAPORE
AstraZeneca AB
Active Ingredients
Documents
Package Inserts
Marcain Injection 0.5% (Polyamp Duofit) PI.pdf
Approved: December 26, 2018
