Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
AEROSOL, METERED
**4.2 Posology and Method of Administration** **4.2.1 General** **ZENHALE** should be administered by oral inhalation only. After each dose, patients should be advised to rinse their mouth with water and spit out the contents without swallowing. The cap from the mouthpiece of the actuator should be removed before using **ZENHALE**. The **ZENHALE** canister should only be used with the **ZENHALE** actuator. The **ZENHALE** actuator should not be used with any other inhalation drug product. Actuators from other products should not be used with the **ZENHALE** canister. **4.2.2 Dose** **ZENHALE** should be administered as two inhalations twice daily (morning and evening) by oral inhalation. When choosing the starting dosage strength of **ZENHALE**, consider the patient’s disease severity, based on their previous asthma therapy, including the inhaled corticosteroid dosage, as well as the patient’s current control of asthma symptoms and risk of future exacerbation. For patients whose asthma is currently controlled, the recommended dose for **ZENHALE** treatment based on prior asthma therapy is provided in the table below. 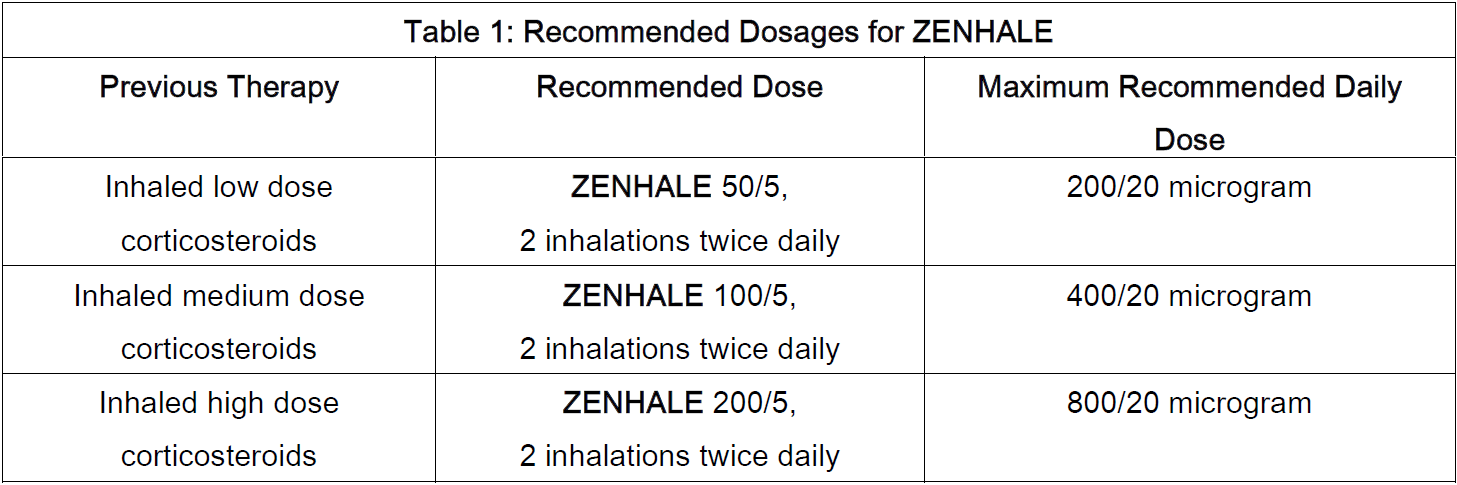 For patients who have not previously received inhaled glucocorticosteroids, but whose disease severity warrants initiation of treatment with two maintenance therapies, depending upon asthma severity, the recommended starting dose is **ZENHALE** 50/5, **ZENHALE** 100/5, or **ZENHALE** 200/5, two inhalations twice daily. **4.2.3 Duration of Treatment** Long-term, twice-daily maintenance treatment for asthma. **4.2.4 Method of Administration** **ZENHALE** should be administered by oral inhalation only. **4.2.5 Other Important Information** The maximum daily recommended dose is two inhalations of **ZENHALE** 200/5 twice daily for patients 12 years of age and older. If symptoms arise between doses, an inhaled short-acting beta2-agonist should be taken for immediate relief. Patients should be regularly reassessed by a doctor. If a previously effective dosage regimen of **ZENHALE** fails to provide adequate control of asthma, the therapeutic regimen should be re-evaluated and additional therapeutic options, e.g., replacing the current strength of **ZENHALE** with a higher strength, adding additional inhaled corticosteroid, or initiating oral corticosteroids, should be considered. Patients should be made aware that for optimum benefit, **ZENHALE** should be taken regularly, even when they are asymptomatic. Rescue medications only need to be taken to relieve acute asthma symptoms. If a previously effective dosage regimen of **ZENHALE** fails to provide adequate control of asthma, medical advice should be sought and therapy reassessed, as this may indicate a worsening of the underlying condition. **Missed Dose** If a dose is missed, the patient should be instructed to take the next dose as soon as they remember unless it is near to the time for the next dose, at which time they should wait until the next dose is due. The patient should be instructed not to double the dose. **Geriatrics (≥65 years of age):** Based on available data for **ZENHALE** or its active components, no adjustment of **ZENHALE** in geriatric patients is warranted. **Pediatrics (<12 years of age):** The safety and efficacy of **ZENHALE** have not been established in children less than 12 years of age.
RESPIRATORY (INHALATION)
Medical Information
**4.1 Therapeutic Indications** **ZENHALE**, administered twice daily, is indicated for the maintenance treatment of asthma, in adults and children 12 years of age and older with reversible obstructive airway disease, whose asthma cannot be adequately controlled on asthma controller medications. **ZENHALE** is not indicated for patients whose asthma can be managed by occasional use of a rapid onset, short duration, inhaled beta2-agonist or for patients whose asthma can be successfully managed by inhaled corticosteroids along with occasional use of a rapid onset, short duration, inhaled beta2-agonist. **ZENHALE** should be used for patients not adequately controlled with inhaled corticosteroids and “as needed” inhaled short-acting beta2-agonists or whose disease severity clearly warrants initiation of treatment with two maintenance therapies. **ZENHALE** may also be used in patients already adequately controlled on both inhaled corticosteroid and long-acting beta2-agonist. **ZENHALE** contains a long-acting beta2-agonist and should not be used as a rescue medication. To relieve acute asthmatic symptoms, a rapid onset, short duration inhaled bronchodilator (e.g., salbutamol) should be used. Once asthma control is achieved and maintained, assess the patient at regular intervals and step down therapy (e.g., discontinue **ZENHALE**) if possible without loss of asthma control, and maintain the patient on a long-term asthma control medication, such as an inhaled corticosteroid. Do not use **ZENHALE** for patients whose asthma is adequately controlled on low or medium dose inhaled corticosteroids.
**5.1 Contraindications** Patients with known hypersensitivity to mometasone furoate, formoterol fumarate or to any of the excipients.
R03AK07
formoterol and budesonide
Manufacturer Information
ORGANON SINGAPORE PTE. LTD.
Kindeva Drug Delivery Limited
Active Ingredients
Documents
Package Inserts
Zenhale PI.pdf
Approved: June 12, 2023
