Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Capecitabine should only be prescribed by a qualified physician experienced in the utilisation of anti-neoplastic agents. Capecitabine tablets should be swallowed with water within 30 minutes after a meal. Capecitabine tablets should not be crushed or cut. Treatment should be discontinued if progressive disease or intolerable toxicity is observed. Standard and reduced dose calculations according to body surface area for starting doses of capecitabine of 1250 mg/m2 and 1000 mg/m2 are provided in tables 1 and 2, respectively. Recommended posology (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): Monotherapy _Colon, colorectal and breast cancer_ Given as single agent, the recommended starting dose for capecitabine in the adjuvant treatment of colon cancer, in the treatment of metastatic colorectal cancer or of locally advanced or metastatic breast cancer is 1250 mg/m2 administered twice daily (morning and evening; equivalent to 2500 mg/m2 total daily dose) for 14 days followed by a 7-day rest period. Adjuvant treatment in patients with stage III colon cancer is recommended for a total of 6 months. Combination therapy _Colon, colorectal and gastric cancer_ In combination treatment, the recommended starting dose of capecitabine should be reduced to 1000 mg/m2 administered twice daily for 2 weeks followed by a 7-day rest period. The inclusion of biological agents in a combination regimen has no effect on the starting dose of capecitabine. Premedication to maintain adequate hydration and anti-emesis according to the cisplatin and oxaliplatin product information should be started prior to cisplatin administration for patients receiving the capecitabine plus cisplatin or oxaliplatin combination. Adjuvant treatment in patients with stage III colon cancer is recommended for a total of 6 months. _Breast cancer_ In combination with docetaxel, the recommended starting dose of capecitabine in the treatment of metastatic breast cancer is 1250 mg/m2 twice daily for 14 days followed by a 7-day rest period, combined with docetaxel at 75 mg/m2 as a 1 hour intravenous infusion every 3 weeks. Pre-medication with an oral corticosteroid such as dexamethasone according to the docetaxel summary of product characteristics should be started prior to docetaxel administration for patients receiving the capecitabine plus docetaxel combination. Capecitabine Dose Calculations 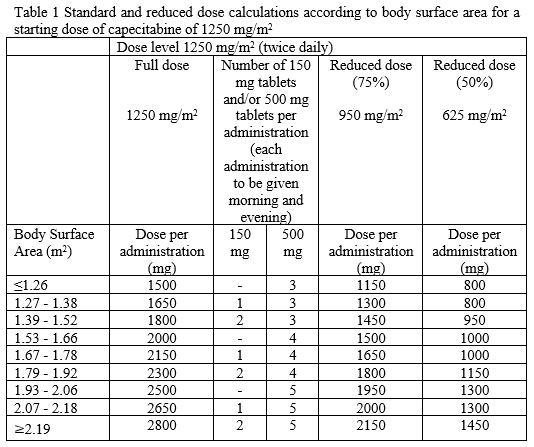 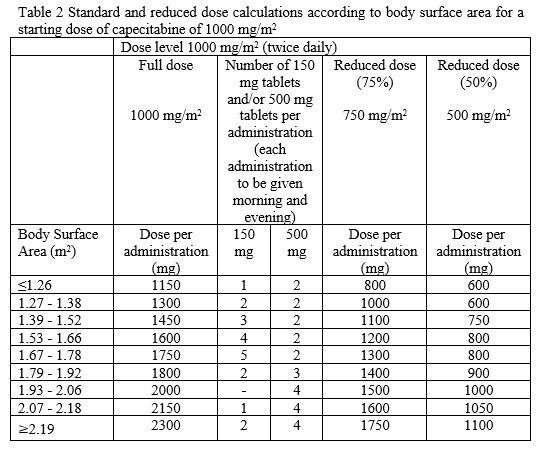 Posology adjustments during treatment _General_ Toxicity due to capecitabine administration may be managed by symptomatic treatment and/or modification of the dose (treatment interruption or dose reduction). Once the dose has been reduced, it should not be increased at a later time. For those toxicities considered by the treating physician to be unlikely to become serious or life-threatening, e.g. alopecia, altered taste, nail changes, treatment can be continued at the same dose without reduction or interruption. Patients taking capecitabine should be informed of the need to interrupt treatment immediately if moderate or severe toxicity occurs. Doses of capecitabine omitted for toxicity are not replaced. The following are the recommended dose modifications for toxicity: 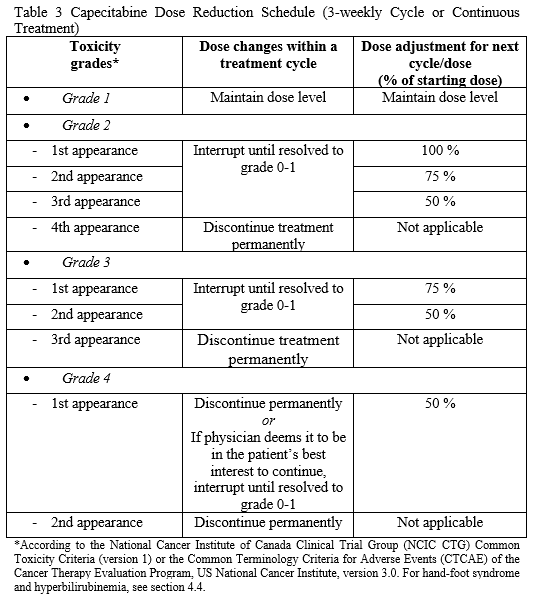 _Haematology_ Patients with baseline neutrophil counts of <1.5 x 109/L and/or thrombocyte counts of <100 x 109/L should not be treated with Capecitabine Alvogen. If unscheduled laboratory assessments during a treatment cycle show that the neutrophil count drops below 1.0 x 109/L or that the platelet count drops below 75 x 109/L, treatment with capecitabine should be interrupted. _Dose modifications for toxicity when capecitabine is used in combination with other agents_ Dose modifications for toxicity when capecitabine is used in combination with other therapies should be made according to Table 3 above for capecitabine and according to the appropriate Prescribing Information for the other agent(s). At the beginning of a treatment cycle, if a treatment delay is indicated for either capecitabine or the other agent(s), then administration of all agents should be delayed until the requirements for restarting all drugs are met. During a treatment cycle for those toxicities considered by the treating physician not to be related to capecitabine, capecitabine should be continued and the dose of the other agent adjusted according to the appropriate Prescribing Information. If the other agent(s) have to be discontinued permanently, capecitabine treatment can be resumed when the requirements for restarting capecitabine are met. This advice is applicable to all indications and to all special populations. Posology adjustments for special populations: _Hepatic impairment_ In patients with mild to moderate hepatic impairment due to liver metastases, no starting dose adjustment is necessary. However, such patients should be carefully monitored. Patients with severe hepatic impairment have not been studied. _Renal impairment_ Capecitabine is contraindicated in patients with severe renal impairment (creatinine clearance below 30 ml/min \[Cockcroft and Gault\] at baseline). The incidence of grade 3 or 4 adverse reactions in patients with moderate renal impairment (creatinine clearance 30–50 ml/min at baseline) is increased compared to the overall population. In patients with moderate renal impairment at baseline, a dose reduction to 75% for a starting dose of 1250 mg/m2 is recommended. In patients with mild renal impairment (creatinine clearance 51–80 ml/min at baseline) no adjustment of the starting dose is recommended. Careful monitoring and prompt treatment interruption is recommended if the patient develops a grade 2, 3 or 4 adverse event during treatment and subsequent dose adjustment as outlined in Table 3 above. If the calculated creatinine clearance decreases during treatment to a value below 30 ml/min, capecitabine should be discontinued. These dose adjustment recommendations for renal impairment apply both to monotherapy and combination use (see also section “Elderly” below). _Paediatric population_ There is no experience in children (under 18 years). _Elderly_ During capecitabine monotherapy, no adjustment of the starting dose is needed. However, grade 3 or 4 treatment-related adverse reactions were more frequent in patients ≥60 years of age compared to younger patients. When capecitabine was used in combination with other agents, elderly patients (≥65 years) experienced more grade 3 and grade 4 adverse drug reactions, including those leading to discontinuation, compared to younger patients. Careful monitoring of patients ≥60 years of age is advisable. - _In combination with docetaxel:_ an increased incidence of grade 3 or 4 treatment-related adverse reactions and treatment-related serious adverse reactions were observed in patients 60 years of age or more (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients 60 years of age or more, a starting dose reduction of capecitabine to 75% (950 mg/m2 twice daily) is recommended. If no toxicity is observed in patients ≥60 years of age treated with a reduced capecitabine starting dose in combination with docetaxel, the dose of Capecitabine may be cautiously escalated to 1250 mg/m2 twice daily. - _In combination with irinotecan:_ for patients 65 years of age or more, a starting dose reduction of capecitabine to 800 mg/m2 twice daily is recommended.
ORAL
Medical Information
**4.1 Therapeutic indications** Capecitabine is indicated for the adjuvant treatment of patients following surgery of stage III (Dukes’ stage C) colon cancer (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Capecitabine is indicated for the treatment of metastatic colorectal cancer (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Capecitabine is indicated for first-line treatment of advanced gastric cancer in combination with a platinum-based regimen (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Capecitabine in combination with docetaxel (see section 5.1) is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Previous therapy should have included an anthracycline. capecitabine is also indicated as monotherapy for the treatment of patients with locally advanced or metastatic breast cancer after failure of taxanes and an anthracycline-containing chemotherapy regimen or for whom further anthracycline therapy is not indicated.
**4.3 Contraindications** - History of severe and unexpected reactions to fluoropyrimidine therapy, - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 or fluorouracil – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_, - Known complete dihydropyrimidine dehydrogenase (DPD) deficiency (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), - During pregnancy and lactation, - In patients with severe leucopenia, neutropenia, or thrombocytopenia, - In patients with severe hepatic impairment, - In patients with severe renal impairment (creatinine clearance below 30 ml/min), - Treatment with sorivudine or its chemically related analogues, such as brivudine (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), - If contraindications exist to any of the agents in the combination regimen, that agent should not be used.
L01BC06
capecitabine
Manufacturer Information
LOTUS INTERNATIONAL PTE. LTD.
Remedica Ltd
Active Ingredients
Documents
Package Inserts
1.4.3 PI 052021_capecitabine alvogen fct 150mg & 500mg-clean.doc
Approved: August 30, 2021
