Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage and administration** **Method of Administration** Foscarnet should be administered by the intravenous route only, either by a central venous line or in a peripheral vein. When peripheral veins are used, the solution of foscarnet 24 mg/ml must be diluted with dextrose 5% or normal saline to a concentration of 12 mg/ml immediately prior to administration. The solution of foscarnet 24 mg/ml may be given without dilution via a central vein. **Adults** _Induction therapy_ Foscarnet can be administered either as a continuous infusion over 24 hours or as intermittent infusions every 8 hours at a dose of 60 mg/kg in patients with normal renal function. (see dosing chart below). When foscarnet is administered as a continuous infusion the treatment is started with an intravenous infusion of 20mg/kg body weight over a period of 30 minutes followed by a continuous intravenous infusion at a dose determined by renal function as assessed by estimated creatinine clearance. When administered as intermittent infusions every 8 hours, the dose of foscarnet should be adjusted to the renal function as assessed by estimated creatinine clearance. The infusion time should not be shorter than 1 hour. _Maintenance therapy_ For maintenance therapy foscarnet is administered seven days a week as a once daily infusion over 2 hours at a dose determined by renal function as assessed by the estimated creatinine clearance, as long as therapy is considered appropriate. In patients with normal renal function the dose range is 90 – 120 mg/kg. The dose recommendations are approximate and final dosing should be based on the clinical situation. **Caution – Do not administer foscarnet by rapid intravenous injection.** 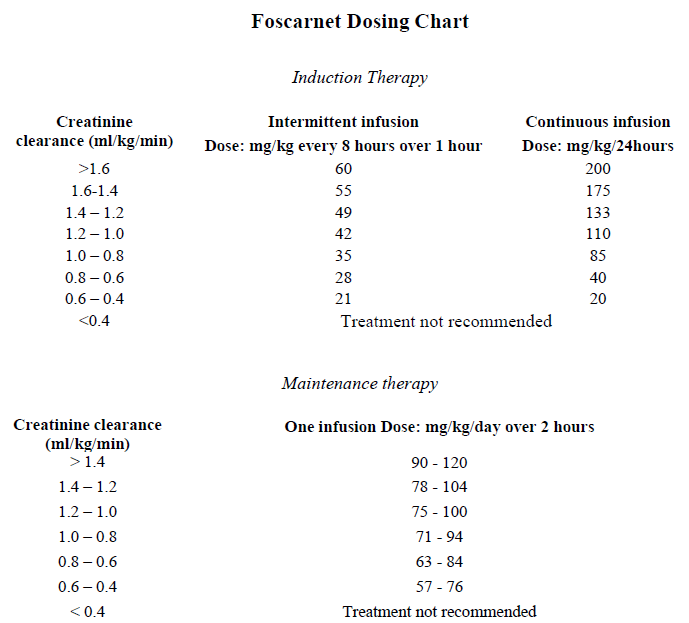 Foscarnet is not recommended for use in patients undergoing haemodialysis as dosage guidelines have not been established. Hydration Renal toxicity can be reduced by adequate hydration of the patient. It is recommended to add 2.5 litres over 24 hours when foscarnet is given as continuous infusion and to add 0.5 – 1.0 litre of normal saline to each infusion when on intermittent therapy. In compliant patients, oral hydration with similar hydration regimens has been used. Clinically dehydrated patients should have their condition corrected before initiating Foscavir therapy. Duration of Treatment An initial induction treatment period of 2–3 weeks is recommended, depending on the clinical response followed by maintenance therapy for as long as considered appropriate. **Elderly:** As for adults. **Paediatric population:** The safety and efficacy of foscarnet in children have not been established. Please refer to Description, and Precautions and Warnings sections – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **Renal or hepatic insufficiency:** The dose must be reduced in patients with renal insufficiency according to the creatinine clearance level as described in the table above. Dose adjustment is not required in patients with hepatic insufficiency.
INTRAVENOUS
Medical Information
**Indication** Cytomegalovirus (CMV) retinitis in patients with the acquired immunodeficiency syndrome (AIDS).
**Contraindication** Hypersensitivity to foscarnet.
J05AD01
foscarnet
Manufacturer Information
LINK HEALTHCARE SINGAPORE PTE. LTD.
Fresenius Kabi Austria GmbH
Active Ingredients
Documents
Package Inserts
1.4.3-package-insert-clean.pdf
Approved: March 2, 2021
