Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2. Posology and method of administration** **Dosage** Neutropenic patients with signs of infection (e.g., fever) should receive immediate empirical antibiotic therapy before laboratory results are available. _**Adults and children over 12 years**_ The usual dosage for adults and children over 12 years with normal renal function is 4.5 g TAZOCIN given every eight hours. The total daily dose depends on the severity and localization of the infection and can vary from 2.25 g to 4.5 g TAZOCIN administered every six or eight hours. In neutropenia, the recommended dose is 4.5 g TAZOCIN given every six hours in combination with an aminoglycoside. _**Children under the age of 12 years**_ TAZOCIN is only recommended for the treatment of children with neutropenia. For children weighing over 50 kg, follow adult dosing guidance, including the aminoglycoside. For children with normal renal function and weighing less than 50 kg, the dose should be adjusted to 90 mg/kg (80 mg piperacillin/10 mg tazobactam) administered every six hours in combination with an aminoglycoside. **Until further experience is available, TAZOCIN should not be used in children who do not have neutropenia.** _**Hospitalized children with intra-abdominal infection**_ For children aged 2 to 12 years, weighing up to 40 kg, and with normal renal function, the recommended dose is 112.5 mg/kg (100 mg piperacillin/12.5 mg tazobactam) every 8 hours. For children aged 2 to 12 years, weighing over 40 kg, and with normal renal function, follow the adult dosing guidance, i.e., 4.5 g (4 g piperacillin/0.5 g tazobactam) every 8 hours. The duration of therapy should be guided by the severity of the infection and the patient’s clinical and bacteriological progress. _**Elderly**_ TAZOCIN may be used at the same dose levels as adults except in cases of renal impairment (see below): _**Renal insufficiency in adults and children weighing >50 kg**_ In adults and children weighing >50 kg with renal insufficiency, the intravenous dose should be adjusted to the degree of actual renal impairment. The suggested daily doses are as follows: 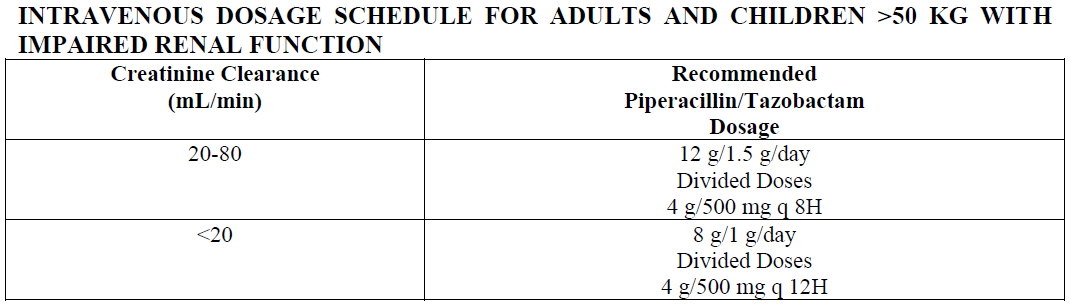 For patients on hemodialysis, the maximum daily dose is 8 g/1 g TAZOCIN. In addition, because hemodialysis removes 30%–50% of piperacillin in 4 hours, one additional dose of 2 g/250 mg TAZOCIN should be administered following each dialysis period. For patients with renal failure and hepatic insufficiency, measurement of serum levels of TAZOCIN will provide additional guidance for adjusting dosage. _**Renal insufficiency in adults and children weighing <50 kg**_ In adults and children weighing <50 kg with renal insufficiency, the intravenous dose should be adjusted to the degree of actual renal impairment. The suggested daily doses are as follows:  For children weighing <50 kg on hemodialysis, the recommended dose is 45 mg/kg q 8H. The pharmacokinetics of TAZOCIN have not been studied in pediatric patients with renal impairment. Each patient must be monitored closely for signs of drug toxicity. Drug dose and interval dose should be adjusted accordingly. In patients with renal insufficiency or hemodialysis patients, intravenous dosages and administration intervals should be adjusted to the degree of renal function impairment. **Duration of therapy** Therapy is recommended for a minimum of 5 days and maximum of 14 days, considering that dose administration should continue at least 48 hours after the resolution of clinical signs and symptoms or fever. **Administration** TAZOCIN must be given by slow intravenous injection (over at least 3–5 minutes) or by slow intravenous infusion (e.g., over 20–30 minutes). TAZOCIN should not be mixed with other drugs in a syringe or infusion bottle since compatibility has not been established. In particular, whenever TAZOCIN is used concurrently with another antibiotic, especially an aminoglycoside (with the exceptions shown below), TAZOCIN must not be mixed in intravenous solutions or administered concurrently due to physical incompatibility. _**Co-administration of piperacillin/tazobactam with aminoglycosides**_ Due to the _in vitro_ inactivation of the aminoglycoside by the β-lactam antibiotics, piperacillin/tazobactam and the aminoglycoside are recommended for separate administration. Piperacillin/tazobactam and the aminoglycoside should be reconstituted and diluted separately when concomitant therapy with aminoglycosides is indicated. The following compatibility information does not apply to the piperacillin/tazobactam formulation not containing EDTA. In circumstances where co-administration is preferred, the reformulated piperacillin/tazobactam containing EDTA supplied in vials is compatible for simultaneous co-administration via Y-site infusion only with the following aminoglycosides under the following conditions: 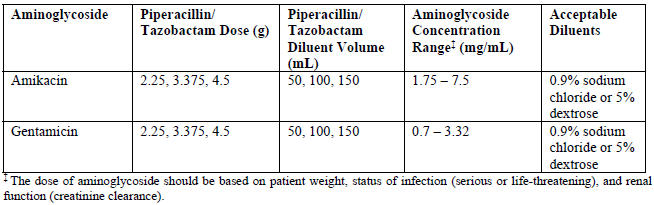 Compatibility of piperacillin/tazobactam with other aminoglycosides has not been established. Only the concentration and diluents for amikacin and gentamicin with the dosages of piperacillin/tazobactam listed in the table above have been established as compatible for co-administration via Y-site infusion. Simultaneous co-administration via Y-site infusion in any manner other than listed above may result in inactivation of the aminoglycoside by piperacillin/tazobactam.
INTRAVENOUS
Medical Information
**4.1. Therapeutic indications** TAZOCIN is indicated for the treatment of the following systemic and/or local bacterial infections in which susceptible organisms have been detected or are suspected: - Lower respiratory tract infections; urinary tract infections (complicated and uncomplicated); intra-abdominal infections; skin and skin structure infections; bacterial septicemia. - Polymicrobial infections: TAZOCIN is indicated for polymicrobial infections including those where aerobic and anaerobic organisms are suspected (intra-abdominal, skin and skin structure, lower respiratory tract). TAZOCIN, in combination with an aminoglycoside, is indicated for bacterial infections in neutropenic adults or children. **Children Under the Age of 12 Years** In hospitalized children aged 2 to 12 years, TAZOCIN is indicated for the treatment of intra-abdominal infections including appendicitis complicated by rupture or abscess, peritonitis, and biliary infections. It has not been evaluated in this indication for pediatric patients below the age of 2 years. Whilst TAZOCIN is indicated only for the conditions listed above, infections caused by piperacillin susceptible organisms are also amendable to TAZOCIN treatment due to its piperacillin content. Therefore, the treatment of mixed infections caused by piperacillin susceptible organisms and β-lactamase producing organisms susceptible to TAZOCIN should not require the addition of another antibiotic. TAZOCIN is particularly useful in the treatment of mixed infections and in presumptive therapy prior to the availability of the results of sensitivity tests because of its broad spectrum of activity. TAZOCIN acts synergistically with aminoglycosides against certain strains of _Pseudomonas aeruginosa_. Combined therapy has been successful, especially in patients with impaired host defences. Both drugs should be used in full therapeutic doses. As soon as results of culture and susceptibility tests become available, antimicrobial therapy should be adjusted if necessary. _Note: For associated bacteremia due to extended-beta-lactamase (ESBL) producing organisms, see section 5.1_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
**4.3. Contraindications** Hypersensitivity to any of the β-lactams (including penicillins and cephalosporins) or to β-lactamase inhibitors.
J01CR05
piperacillin and beta-lactamase inhibitor
Manufacturer Information
PFIZER PRIVATE LIMITED
Wyeth Lederle S.r.l
PFE Wyeth Ayerst (Asia) LLC, Taiwan Branch, Hsinchu Plant
Active Ingredients
Documents
Package Inserts
Tazocin Injection PI.pdf
Approved: February 22, 2023
