Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**Posology and method of administration** ENDOXAN should only be administered by experienced oncologists. The dosage must be adapted to each patient individually. Unless otherwise prescribed the following dosages are recommended: ENDOXAN 200 mg/500 mg/1 g, injection vials: - for continuous treatment in adults and children 3 to 6 mg/kg body weight daily (equivalent to 120 to 240 mg/m2 body surface) - for intermittent treatment 10 to 15 mg/kg body weight (equivalent to 400 to 600 mg/m2 body surface) at intervals of 2 to 5 days - for high-dose intermittent treatment, e.g. 20 to 40 mg/kg body weight (equivalent to 800 to 1600 mg/m2 body surface) and higher doses (e.g. for conditioning prior to bone-marrow transplantation) at intervals of 21 to 28 days. **Preparation of the solution** To prepare a solution for injection, the respective amount of physiological saline is added to the dry substance: 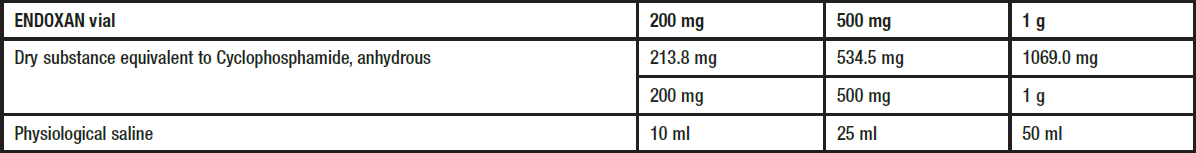 The substance dissolves readily if the vials are vigorously shaken after addition of the solvent. If the substance fails to dissolve immediately and completely, it is advisable to allow the vial to stand for a few minutes. The solution is suitable for intravenous administration which preferably should be conducted as an infusion. For short term intravenous infusion, the prepared ENDOXAN solution is added to Ringer's solution, saline or dextrose solution for a total volume of e.g. 500 ml. The duration of infusion may range from 30 minutes to 2 hours, depending on the volume. The dose recommendations given mainly apply to the treatment with cyclophosphamide as a monotherapy. In combination with other cytostatics of similar toxicity, a dose reduction or extension of the therapy-free intervals may be necessary. _Recommendations for dose reduction in patients with myelosuppression_ 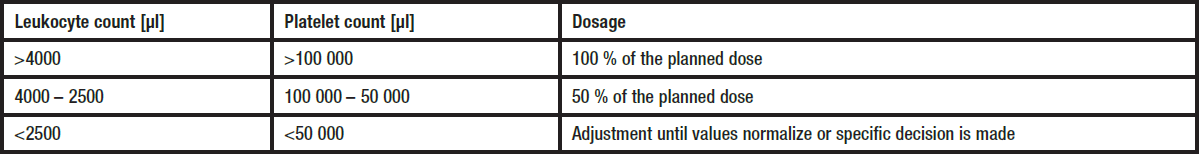 _Recommendations for dose adjustment in patients with hepatic and renal insufficiency_ Severe hepatic- or renal insufficiency requires a dose reduction. A dose reduction of 25 % for serum bilirubin from 3.1 to 5 mg/100 ml and of 50 % for a glomerular filtration rate below 10 ml/minute is recommended. Cyclophosphamide is dialysable. ENDOXAN 200 mg/500 mg/1 g, injection vials Duration of therapy and intervals will depend on the indication, the applied combination chemotherapy schedule, the patient's general state of health, the laboratory parameters and the recovery of blood cell counts. Attention should be paid to adequate hydration as well as to the administration of the UROPROTECTOR UROMITEXAN.
INTRAVENOUS
Medical Information
**Therapeutic Indications** ENDOXAN is used within a combination chemotherapy regimen or as monotherapy in Leukaemias: acute or chronic lymphocytic and myelogenous leukaemias Malignant lymphomas: Hodgkin's disease, non-Hodgkin's lymphomas, plasmacytoma Metastasizing and non-metastasizing malignant solid tumours: ovarian cancer, testicular cancer, breast cancer, small cell lung cancer, neuroblastoma, Ewing's sarcoma Progressive “autoimmune diseases”: e.g. rheumatoid arthritis, psoriatic arthropathy, systemic lupus erythematosus, scleroderma, systemic vasculitides (e.g. with nephrotic syndrome), certain types of glomerulonephritis (e.g. with nephrotic syndrome), myasthenia gravis, autoimmune haemolytic anaemia, cold agglutinin diseases. Immunosuppressive treatment in organ transplantations
**Contraindications** ENDOXAN should not be used in patients with - known hypersensitivity to cyclophosphamide - severely impaired bone-marrow function (particular in patients who have been pre-treated with cytotoxic agents and/or radiotherapy) - inflammation of the bladder (cystitis) - urinary outflow obstructions - active infections Endoxan should not be used in pregnancy and lactation (see pregnancy and lactation – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_)
L01AA01
cyclophosphamide
Manufacturer Information
BAXTER HEALTHCARE (ASIA) PTE LTD
BAXTER ONCOLOGY GMBH
Active Ingredients
Documents
Package Inserts
Endoxan_PI.pdf
Approved: February 14, 2023
