Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, COATED
**4.2 Posology and Method of Administration** For the individual adjustment of dose, the strengths of 25 mg and 50 mg are available. The maximum dose regimen is 50 mg daily for heart failure and 100 mg daily for hypertension. **For Patients with Hypertension:** The recommended starting dose of eplerenone is 50 mg administered once daily. The full therapeutic effect of eplerenone is apparent within 4 weeks. For patients with an inadequate blood pressure response to 50 mg once daily the dosage of eplerenone should be increased to 50 mg twice daily. Higher dosages of eplerenone are not recommended because they have no greater effect on blood pressure than 100 mg and are associated with an increased risk of hyperkalemia. **For Post-myocardial Infarction Heart Failure Patients:** The recommended maintenance dose of eplerenone is 50 mg once daily. Treatment should be initiated at 25 mg once daily and titrated in one step to the target dose of 50 mg once daily preferably within 4 weeks, taking into account the serum potassium level (see Table 1). Eplerenone therapy should usually be started within 3–14 days after an acute myocardial infarction. **For Patients with NYHA Class II (Chronic) Heart Failure:** For chronic heart failure NYHA class II patients, treatment should be initiated at a dose of 25 mg once daily and titrated to the target dose of 50 mg once daily preferably within 4 weeks, taking into account the serum potassium level (see Table 1 and section **4.4 Special Warnings and Precautions for Use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **General Considerations:** Patients with a serum potassium of >5.0 mmol/L should not be started on eplerenone (see section **4.3 Contraindications**). Serum potassium should be measured before initiating eplerenone therapy, within the first week and at one month after the start of treatment or dose adjustment. Serum potassium should be assessed as needed periodically thereafter. After initiation, the dose should be adjusted based on the serum potassium level as shown in Table 1. 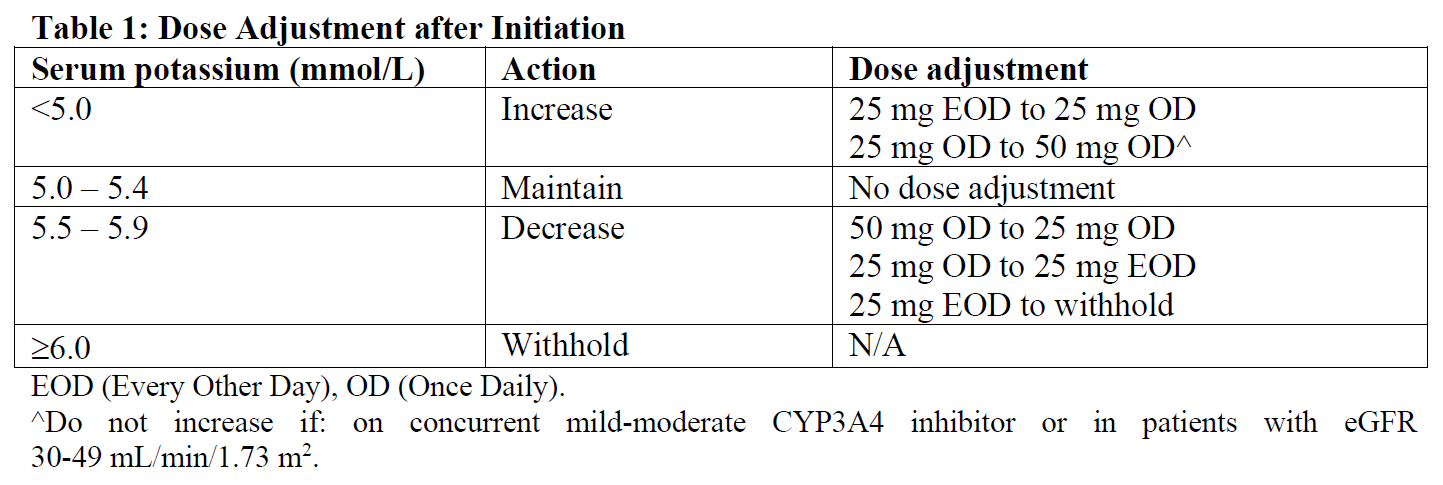 Following withholding eplerenone due to serum potassium ≥6.0 mmol/L, eplerenone can be re-started at a dose of 25 mg every other day when potassium levels have fallen below 5.0 mmol/L. **Food:** Eplerenone may be administered with or without food. **Concomitant CYP3A4 Medications:** In patients receiving mild to moderate CYP3A4 inhibitors, such as amiodarone, diltiazem, erythromycin, saquinavir, verapamil, and fluconazole, dosing should not exceed 25 mg once daily. **Special Populations and Special Considerations for Dosing** **Use in Hepatic Impairment** _Mild-to-Moderate Hepatic Impairment:_ No initial dose adjustment is necessary (see sections **4.3 Contraindications** and **4.4 Special Warnings and Precautions for Use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Use in Renal Impairment** **For post-myocardial infarction heart failure patients:** No initial dose adjustment is required in patients with mild renal impairment (creatinine clearance ≥50 mL/min). The rates of hyperkalemia increase with declining renal function. Periodic monitoring of serum potassium with dose adjustment according to Table 1 is recommended (see section **4.4 Special Warnings and Precautions for Use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There is no experience in patients with moderate to severe renal impairment (creatinine clearance <50 mL/min) or Type 2 Diabetes with microalbuminuria. The use of eplerenone in these patients is contraindicated. **For patients with NYHA class II (chronic) heart failure:** No initial dose adjustment is required in patients with mild renal impairment (eGFR ≥50 mL/min/1.73 m2). The rates of hyperkalemia increase with declining renal function. Periodic monitoring of serum potassium is recommended (see section **4.4 Special Warnings and Precautions for Use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) and doses adjusted according to Table 1. Patients with moderate renal impairment (eGFR 30 – 49 mL/min/1.73 m2) should be started at 25 mg every other day, and dose should be adjusted based on the potassium level (see Table 1). Periodic monitoring of serum potassium is recommended (see section **4.4 Special Warnings and Precautions for Use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Doses above 25 mg daily have not been studied in patients with eGFR 30–49 mL/min/1.73 m2. There is no experience in patients with severe renal impairment eGFR <30 mL/min/1.73 m2. The use of eplerenone in these patients is contraindicated. Eplerenone is not dialyzable. **For patients with hypertension:** For hypertensive patients with moderate-to-severe renal impairment or Type 2 diabetes with microalbuminuria, see sections **4.3 Contraindications** and **4.4 Special Warnings and Precautions for Use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **Use in the Elderly** No initial adjustment of the dose is required in the elderly patients (see section **4.4 Special Warnings and Precautions for Use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Use in Children** Safety and efficacy of eplerenone has not been studied in pediatric patients with heart failure. Eplerenone has not been studied in hypertensive patients less than 4 years old and the study in older pediatric patients did not demonstrate efficacy. Currently available data are described in section **5.1 Pharmacodynamic Properties** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
ORAL
Medical Information
**4.1 Therapeutic Indications** **Hypertension** Eplerenone is indicated for the treatment of hypertension. In these patients, eplerenone may be used alone or in combination with other antihypertensive agents. **Heart Failure – Post-Myocardial Infarction (MI)** Eplerenone is indicated, in addition to standard therapy, to reduce the risk of cardiovascular mortality and cardiovascular hospitalization in stable patients with left ventricular dysfunction (left ventricular ejection fraction \[LVEF\] ≤40%) and clinical evidence of heart failure after recent MI. **New York Heart Association (NYHA) Class II (Chronic) Heart Failure** Eplerenone is indicated, in addition to standard optimal therapy to reduce the risk of cardiovascular mortality and hospitalization in heart failure adult patients with NYHA class II (chronic) heart failure and left ventricular systolic dysfunction (LVEF ≤30% or LVEF ≤35% in addition to QRS duration of >130 msec) (see section **5.1 Pharmacodynamic Properties** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** Eplerenone is contraindicated in all patients with the following: - hypersensitivity to eplerenone or any component of this medication - clinically significant hyperkalemia or with conditions associated with hyperkalemia - serum potassium level >5.0 mmol/L (mEq/L) at initiation - moderate to severe renal impairment (creatinine clearance <50 mL/min) in post-MI heart failure (based on Eplerenone Post-acute Myocardial Infarction Heart failure Efficacy and Survival Study \[EPHESUS\], see section **5.1 Pharmacodynamic Properties** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - severe renal impairment (eGFR <30 mL/min/1.73 m2) in NYHA class II (chronic) heart failure (based on Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure \[EMPHASIS-HF\] study, see section **5.1 Pharmacodynamic Properties** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - severe hepatic impairment (Child-Pugh Class C) - concomitant use with potassium-sparing diuretics or potent inhibitors of CYP450 3A4, such as ketoconazole, itraconazole, and ritonavir (see section **4.5 Interaction with Other Medicinal Products and Other Forms of Interaction** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Eplerenone is also contraindicated in patients with HYPERTENSION and the following: - Type 2 diabetes with microalbuminuria - serum creatinine >2.0 mg/dL (or >177 micromole/L) in males, or >1.8 mg/dL (or >159 micromole/L) in females - concomitant use with potassium supplements
NIL
xnil
Manufacturer Information
VIATRIS PRIVATE LIMITED
Pfizer Pharmaceuticals LLC – Vega Baja
Active Ingredients
Documents
Package Inserts
INSPRA TABLETS PI.pdf
Approved: July 1, 2021
