Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** **Dosage regimen** Unless otherwise prescribed, the following daily doses are recommended for: **Adults** 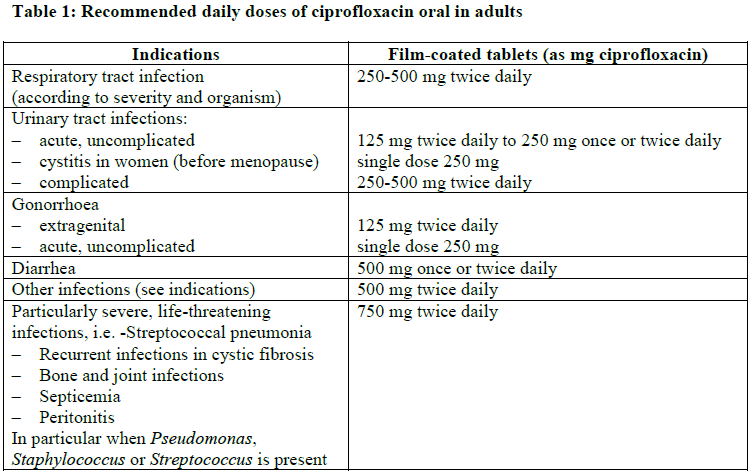 **Missed dose** If a dose is missed, it should be taken as soon as the patient remembers and then treatment should be continued as prescribed. Double doses should not be taken to compensate for a missed dose. **Additional information on special patient populations** **Geriatric patients (> 65 years)** Elderly patients should receive a dose as low as possible depending on the severity of their illness and the creatinine clearance (see also _‘Patients with renal and hepatic impairment’_). **Children**: contraindicated. **Patients with renal and hepatic impairment** **Adults** **Patients with renal impairment**  **Patients with renal impairment on hemodialysis** For patients with creatinine clearance between 30 and 60 ml/min/1.73m2 (moderate renal impairment) or serum creatinine concentration between 1.4 and 1.9 mg/100 ml, the maximum daily dose of ciprofloxacin should be 1000 mg for oral administration. For patients with creatinine clearance less than 30 ml/min/1.73m2 (severe renal impairment) or serum creatinine concentration equal or higher than 2.0 mg/100 ml, the maximum daily dose of ciprofloxacin should be 500 mg for oral administration. **Patients with renal impairment on continuous ambulatory peritoneal dialysis (CAPD)** The maximum daily oral dose of ciprofloxacin should be 500 mg. **Patients with hepatic impairment** In patients with hepatic impairment, no dose adjustment is required. **Patients with renal and hepatic impairment** For patients with creatinine clearance between 30 and 60 ml/min/1.73m2 (moderate renal impairment) or serum creatinine concentration between 1.4 and 1.9 mg/100 ml, the maximum daily oral dose of ciprofloxacin should be 1000 mg. For patients with creatinine clearance less than 30 ml/min/1.73m2 (severe renal impairment) or serum creatinine concentration equal or higher than 2.0 mg/100 ml, the maximum daily oral dose of ciprofloxacin should be 500 mg. **Method of administration** For oral use. Tablets are to be swallowed whole with a small amount of fluid. They can be taken independent of mealtimes. If the tablets are taken on an empty stomach, the active substance is absorbed more rapidly. In this case, ciprofloxacin tablets should not be taken concurrently with dairy products or with mineral-fortified drinks alone (e.g., milk, yoghurt, calcium-fortified orange juice) (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If the patient is unable to take the ciprofloxacin tablets because of the severity of the illness or for other reasons (e.g., patients on enteral nutrition), it is recommended to commence the therapy with an intravenous form of ciprofloxacin. After intravenous administration, the treatment can be continued orally. **Duration of treatment** The duration of treatment depends on the severity of the illness and on the clinical and bacteriological course. It is essential to continue therapy for at least 3 days after disappearance of the fever or of the clinical symptoms. - Mean duration of treatment: 1 day for acute uncomplicated gonorrhoea and cystitis - up to 7 days for infections of the kidneys, urinary tract and abdominal cavity - over the entire period of the neutropenic phase in patients with weakened body defences - a maximum of 2 months in osteomyelitis - and 7–14 days in all other infections In streptococcal infections, the treatment must last at least 10 days because of the risk of late complications. Infections caused by _Chlamydia_ spp. should also be treated for a minimum of 10 days.
ORAL
Medical Information
**4.1 Therapeutic indications** - Uncomplicated and complicated infections caused by ciprofloxacin sensitive pathogens. - Infections of the respiratory tract: Ciprofloxacin can be regarded as an advisable treatment for pneumonias caused by _Klebsiella_ spp., _Enterobacter spp_., _Proteus_ spp., _E. coli_, _Pseudomonas_, _Haemophilus_ spp., _Moraxella Catarrhalis_, _Legionella_ spp. and _Staphylococcus_. - Infections of the middle ear (otitis media), of the paranasal sinuses (sinusitis), especially if these are caused by Gram-negative organisms including _Pseudomonas_ or by _Staphylococcus_. - Infections of the eyes. - Infections of the kidneys and/or the efferent urinary tract. - Infections of the genital organs, including adnexitis, gonorrhoea, prostatitis. - Infections of the abdominal cavity (e.g., infections of the gastrointestinal tract or of the biliary tract, peritonitis). - Infections of the skin and soft tissue. - Infections of the bones and joints. - Sepsis. - Infections or imminent risk of infection (prophylaxis) in patients whose immune system has been weakened (e.g., patients on immunosuppressants or have neutropenia). - Selective intestinal decontamination in immunosuppressed patients. Consideration should be given to available official guidance on the appropriate use of antibacterial agents.
**4.3 Contraindications** Hypersensitivity to the active substance, to other quinolones or to any of the excipients (see section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Concomitant administration of ciprofloxacin and tizanidine (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Ciprofloxacin must not be prescribed for children, adolescents, since there is no experience on the drug's safety in these patient groups and since, on the basis of animal studies, it is not entirely improbable that the drug could cause damage to articular cartilage in the immature organism.
J01MA02
ciprofloxacin
Manufacturer Information
SANDOZ SINGAPORE PTE. LTD.
Salutas Pharma GmbH
Hexal AG
S.C. Sandoz S.R.L.
Active Ingredients
Documents
Package Inserts
CIPRO Package Insert.pdf
Approved: December 11, 2020
