Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Posology _Recommended dosage for intravenous administration_ The following doses can be adjusted depending on the severity of the infection: 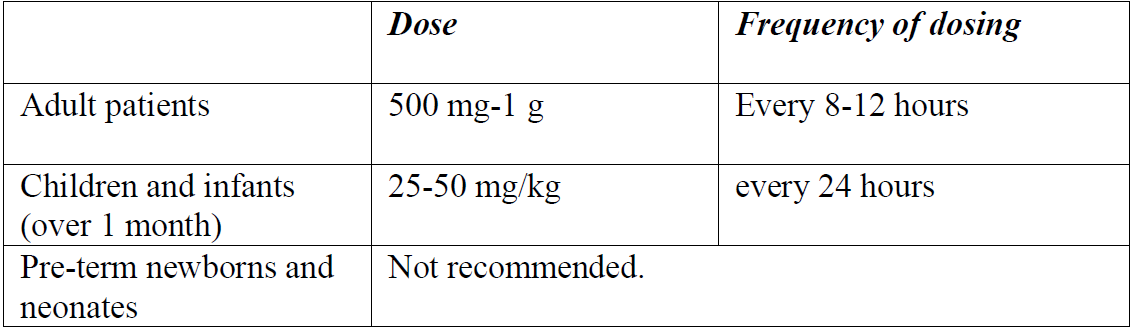 The safety of cefazolin in pre-term newborns and neonates has not been established. Therefore, the use of cefazolin is not recommended. _Recommended dosage for intramuscular administration_ The following doses can be adjusted depending on the severity of the infection: 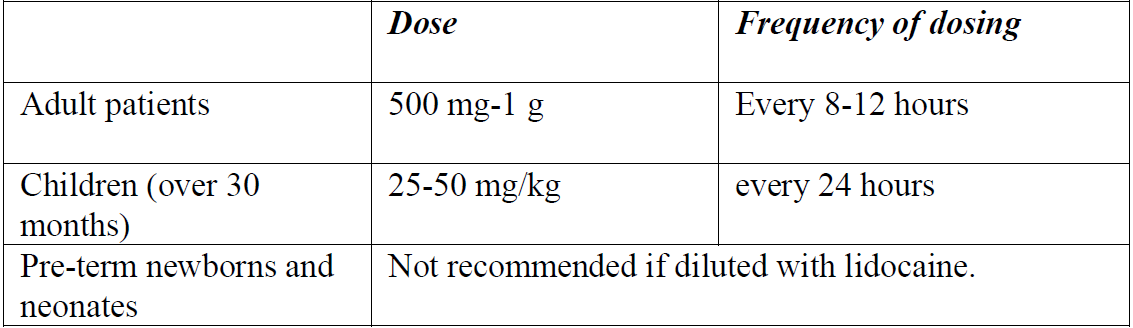 _Recommended dosage in patients with renal impairment_ Severe infections: 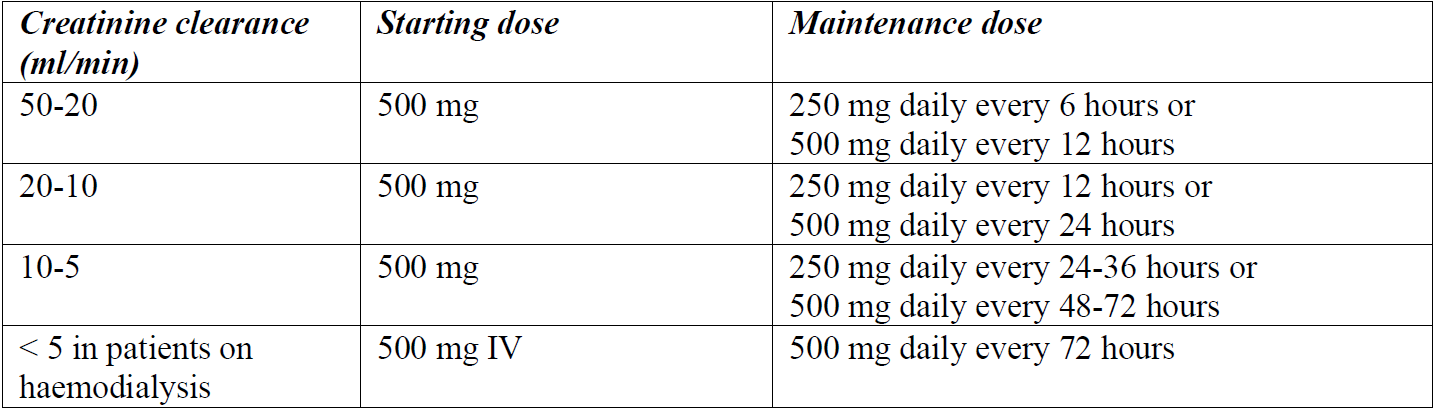 Mild infections: 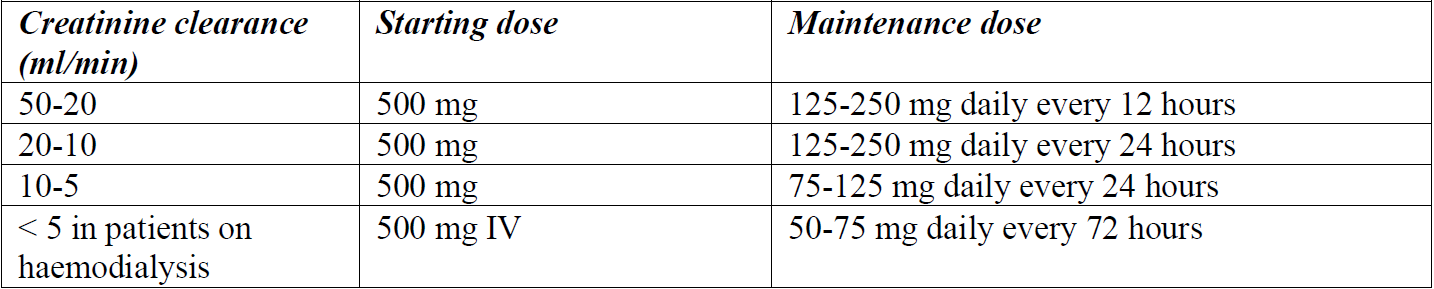 _Prophylaxis of post-operative infections_ Generally, the prophylactic administration of the medicinal product should be short-term – within 24 to 48 hours. - The usual dose is 1 g intravenously administered during the induction of anaesthesia, followed by a dose of 0.5–1 g administered intravenously every 6–8 hours for 24 hours postoperatively. - For operative procedures lasting longer than 2 hours, 0.5–1 g is administered intravenously during surgery. Method of administration CEFAZOLIN ALVOGEN is administered by deep intramuscular injection or by intravenous bolus injection or infusion. The powder for solution for injection or infusion should be initially diluted with 2–3 ml of diluent. The solution should be further reconstituted as follows: _Intramuscular injection:_ The solution is further reconstituted with water for injection (concentration of the solution 250 mg/ml). _Intravenous injection:_ The solution is further reconstituted with water for injection (concentration of the solution 100 mg/ml). CEFAZOLIN ALVOGEN may be administered either by slow intravenous injection over a period of 3 to 5 min directly into a vein or _via_ a drip tube. _Intravenous infusion:_ The solution is further reconstituted with one of the following compatible diluents (concentration of the solution 20 mg/ml): - 0.9% NaCl Injection, - 5% Glucose Injection, - 10% Glucose Injection, - 5% Glucose and 0.9% NaCl Injection, - 5% Glucose and 0.45% NaCl Injection, - 5% Glucose and 0.225% NaCl Injection, - 4% Glucose and 0.18% NaCl Injection, - Compound sodium chloride injection, - Lactated Ringer’s injection. For instructions on reconstitution and dilution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**4.1 Therapeutic indications** CEFAZOLIN ALVOGEN is indicated in adults and children for the treatment of the following infections due to bacteria which are susceptible to cefazolin: - Respiratory tract infections. - Sepsis. - Skin and soft tissue infections. - Endocarditis. - Urinary tract and genital infections. - Bile duct infections. - Bone and joint infections. Other indications that require only intravenous administration: CEFAZOLIN ALVOGEN is indicated for pre-operative prophylaxis of post-operative infections in: - Neurosurgery (craniotomy, liquor derivation). - Cardiac surgery. - Thoracic surgery. - Vascular surgery. - Gastro-intestinal surgery. - Hepatobiliary surgery. - Caesarean section. - Abdominal or vaginal hysterectomy. - Surgery of the head and neck with oropharynx opening. - Orthopaedic surgery. Consideration should be given to official guidance on the appropriate use of antibiotics.
**4.3 Contraindications** Hypersensitivity to the active substance or any other cephalosporins. Pre-term newborns and neonates The safety of cefazolin in pre-term newborns and neonates has not been established. Therefore, the use of cefazolin is not recommended.
J01DB04
cefazolin
Manufacturer Information
LOTUS INTERNATIONAL PTE. LTD.
Sinopharm Zhijun (Shenzhen) Pharmaceutical Co., Ltd
Active Ingredients
Documents
Package Inserts
Cefazolin Singapore 1g PI.pdf
Approved: September 11, 2020
