Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**4.2 Posology and method of administration** For oral use. Deferiprone therapy should be initiated and maintained by a physician experienced in the treatment of patients with thalassaemia. Deferiprone is usually given as 25 mg/kg body weight, orally, three times a day for a total daily dose of 75 mg/kg body weight. Dose per kilogram body weight should be calculated to the nearest 2.5 ml. See table below for recommended doses for body weights at 10 kg increments. Doses above 100 mg/kg/day are not recommended because of the potentially increased risk of adverse reactions (see sections 4.4, 4.8, and 4.9 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There are limited data available on the use of deferiprone in children between 6 and 10 years of age, and no data on deferiprone use in children under 6 years of age. Due to the serious nature of agranulocytosis, that can occur with the use of deferiprone, special monitoring is required for all patients. Caution must be used when the patients’ absolute neutrophil count (ANC) is low, as well as when treating patients with renal insufficiency or hepatic dysfunction. (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Dose table_ To obtain a dose of about 75 mg/kg/day, use the volume of oral solution suggested in the following table for the body weight of the patient. Sample body weights at 10 kg increments are listed. 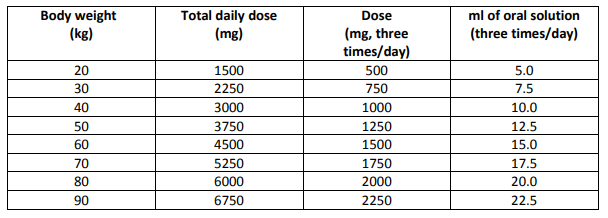
ORAL
Medical Information
**4.1 Therapeutic indications** Ferriprox is indicated for the treatment of iron overload in patients with thalassaemia major when deferoxamine therapy is contraindicated or inadequate.
**4.3 Contraindications** - Hypersensitivity to the active substance or to any of the excipients. - History of recurrent episodes of neutropenia. - History of agranulocytosis. - Pregnancy or breast-feeding (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Due to the unknown mechanism of deferiprone-induced neutropenia, patients must not take medicinal products known to be associated with neutropenia or those that can cause agranulocytosis (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
V03AC02
deferiprone
Manufacturer Information
PHARMAFORTE SINGAPORE PTE LTD
Apotex Inc.- Richmond Hill Site
Active Ingredients
Documents
Package Inserts
Ferriprox Oral Solution PI.pdf
Approved: November 8, 2021
