Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**4.2 Dose and method of administration** **Dosage** _**As a local anaesthetic for infiltration and nerve block:**_ The dosage varies and depends upon the area to be anaesthetised, vascularity of the tissues, number of neuronal segments to be blocked, individual tolerance and the technique of anaesthesia. The lowest dose needed to provide effective anaesthesia should be administered. For specific techniques and procedures, refer to standard textbooks. It is recommended that the dose of lidocaine (lignocaine) at any one time should not exceed 3 mg/kg. However, the dose administered must be tailored to the individual patient and procedure, and the maximum doses here quoted should be used as a guide only. The normal recommended dose of lidocaine (lignocaine) for various anaesthetic procedures in an average, healthy 70 kg adult patient are as follows: 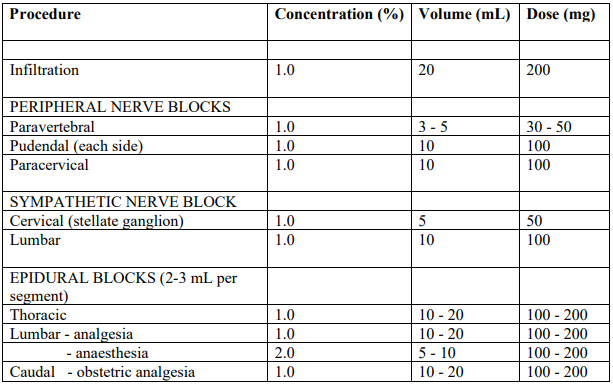 The doses suggested above are only a guide. Toxic levels vary widely between patients so doses should be individualised and blood levels should be monitored. Epidural injections should be administered slowly with frequent aspirations. Subarachnoid and intravascular injections are two of the most serious complications of this technique. If blood or spinal fluid become manifest during aspiration, the needle must be withdrawn and relocated. The technique of epidural anaesthesia should only be attempted by physicians skilled in this area and readiness for emergencies must be ensured. During spinal anaesthesia the positioning of the patient is very important and the patient's pulse and blood pressure should be monitored. During thoracic, lumbar and caudal epidural anaesthesia, a marked fall in blood pressure and/or intercostal paralysis may be seen, possibly due to the use of excessive doses, improper positioning of the patient or accidental disposition of the anaesthetic within the subarachnoid space. Hypotension and bradycardia may occur as a result of sympathetic blockade. For continuous epidural or caudal anaesthesia and paracervical block for obstetric analgesia the maximum dose should not be repeated at intervals of less than 1.5 hours. _Adults:_ The dose should not exceed 200 mg. For spinal anaesthesia, the dose should not exceed 100 mg. _Children:_ For children, a reduced dosage based on body weight or surface area should be used. The dosage should be calculated for each patient individually and modified in accordance with the physician’s experience and knowledge of the patient. Early signs of local anaesthetic toxicity may be difficult to detect in cases where the block is given during general anaesthesia. In order to minimise the possibility of toxic effects, the use of Lignocaine Injection 1% solution is recommended for most anaesthetic procedures involving paediatric patients. The dose should not exceed 3 mg/kg. **_For intravenous use in cardiac arrhythmias:_** Patients with congestive heart failure or cardiogenic shock may require smaller bolus doses. _Adults:_ The usual dose is lidocaine (lignocaine) 50 to 100 mg administered intravenously under ECG monitoring. The dose may be injected at a rate of approximately 25 to 50 mg (2.5 to 5.0 mL of the lidocaine (lignocaine) 1% solution or 1.25 to 2.5 mL of the 2% solution) per minute. A sufficient period of time should be allowed to enable a slow circulation to carry the drug to the site of action. If the initial dose of 50 to 100 mg does not produce the desired response, a second dose may be given after five minutes. No more than 200 to 300 mg of lidocaine (lignocaine) should be administered during a one hour period. Following a single injection in those patients in whom arrhythmia tends to recur and who are incapable of receiving oral antiarrhythmic therapy, intravenous infusions of lidocaine (lignocaine) may be administered at a rate of 1 to 4 mg/minute (20 to 50 mcg/kg/minute). Intravenous infusions must be given under ECG monitoring to avoid potential overdosage and toxicity. The infusion should be terminated as soon as the patient's cardiac rhythm appears to be stable or at the earliest signs of toxicity. It should rarely be necessary to continue the infusion beyond 24 hours. As soon as possible, patients should be changed to an oral antiarrhythmic agent for maintenance therapy. _Paediatrics:_ For children, a reduced dose based on body weight or surface area should be used. It is recommended that the 1% solution be used to minimise the possibility of toxic effects. Experience with lidocaine (lignocaine) is limited. A suggested paediatric dose is a loading dose of 0.5 to 1 mg/kg repeated if necessary up to 3-5 mg/kg, followed by continuous infusions of 10 to 50 micrograms/kg/min. _Geriatrics:_ A reduction in dosage may be necessary for elderly patients, particularly those with compromised cardiovascular and/or hepatic function. **Dosage adjustment** **_Hepatic impairment_** Although lidocaine (lignocaine) is metabolised by the liver, dosage reduction for local anaesthesia is probably not warranted. However, caution should be exercised with repeated doses or prolonged infusion. _**Renal impairment**_ Impairment of renal function is unlikely to affect lidocaine (lignocaine) clearance in the short term (up to 24 hours). However, toxicity due to accumulation may develop with prolonged or repeated administration. Lignocaine Injection is for one dose in one patient only. Discard any remaining contents.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Lidocaine (lignocaine) is indicated for production of local or regional anaesthesia by nerve block, infiltration injection, caudal or other epidural blocks. Treatment or prophylaxis of life-threatening ventricular arrhythmias, including those associated with myocardial infarction, general anaesthesia in patients predisposed to ventricular arrhythmias, digitalis intoxication, or following resuscitation from cardiac arrest.
**4.3 Contraindications** - Allergy or hypersensitivity to local anaesthetics of the amide type or to any excipients. Detection of suspected hypersensitivity by skin testing is of limited value. - Inflammation and/or sepsis at the proposed site of injection and/or in the presence of septicaemia. - Patients with myasthenia gravis, severe shock or impaired cardiac conduction. - Epidural or spinal anaesthesia in patients with - uncorrected hypotension or - coagulation disorders or receiving anticoagulants or - serious diseases of the central nervous system or spinal cord such as meningitis, spinal fluid block, cranial or spinal haemorrhage, tumours, poliomyelitis, syphilis, tuberculosis or metastatic lesions of the spinal cord. - Antiarrhythmic use in patients with - supraventricular arrhythmia or - Stokes-Adams Syndrome or severe degrees of sinoatrial, atrioventricular or intraventricular block unless the patient has an artificial pacemaker. - Lidocaine (lignocaine) suppresses ventricular pacemaker activity and may cause ventricular standstill in such patients. - General contraindications related to epidural anaesthesia, regardless of the local anaesthetic used, should be taken into account.
N01BB02
lidocaine
Manufacturer Information
PFIZER PRIVATE LIMITED
PFIZER (PERTH) PTY LTD
Active Ingredients
Documents
Package Inserts
Lignocaine HCL injection PI.pdf
Approved: June 3, 2021
