Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**4.2 Posology and method of administration** **All patients must be premedicated prior to paclitaxel administration to reduce the risk of severe hypersensitivity reactions. Such premedication may consist of dexamethasone 20 mg orally (or its equivalent) approximately 12 and 6 hours before paclitaxel or 20mg I.V. approximately 30 to 60 minutes before paclitaxel, diphenhydramine 50 mg I.V. (or its equivalent) 30 to 60 minutes prior to paclitaxel and cimetidine (300 mg) or ranitidine (50 mg) I.V. 30 to 60 minutes prior to paclitaxel.** Repeat courses of paclitaxel should not be administered to patients with solid tumours until the neutrophil count is at least 1500 cells/mm3 and the platelet count is at least 100,000 cells/mm3 (<1000 cells/mm3 for patients with Kaposi’s sarcoma). Patients who experience severe neutropenia (<500 cells/mm3) or severe peripheral neuropathy should receive a dosage reduced by 20% for subsequent courses. The incidence of neurotoxicity and the severity of neutropenia increase with dose within a regimen. **Metastatic Carcinoma of the Ovary:** _Combination therapy_: For previously untreated patients, the recommended dosing regimen, given every 3 weeks, is paclitaxel administered intravenously over 3 hours at a dose of 175 mg/m2 followed by a platinum compound. Alternatively, a more myelosuppressive regimen of paclitaxel may also be administered intravenously at a dose of 135 mg/m2 over 24 hours followed by a platinum compound, every 3 weeks. _Single-agent therapy_: In patients previously treated with chemotherapy the recommended regimen is 175 mg/m2 administered intravenously over 3 hours every 3 weeks. **Carcinoma of the Breast:** _Adjuvant therapy_: Paclitaxel 175 mg/m2 administered intravenously over 3 hours every 3 weeks for 4 courses sequentially to standard combination therapy. _Single-agent, first-line therapy after relapse within 6 months of adjuvant therapy_: Paclitaxel 175 mg/m2 administered intravenously over 3 hours every 3 weeks. _Combination, first-line therapy of advanced or metastatic breast cancer_: In combination with trastuzumab, the recommended dose of paclitaxel is 175 mg/m2 administered intravenously over a period of 3 hours, with a 3-week interval between courses. Paclitaxel infusion may be started the day following the first dose of trastuzumab or immediately after the subsequent doses of trastuzumab if the preceding dose of trastuzumab was well tolerated. _Combination, first-line therapy of metastatic breast cancer_: In combination with doxorubicin (50 mg/m2), paclitaxel should be administered 24 hours after doxorubicin. The recommended dose of paclitaxel is 220 mg/m2 administered intravenously over a period of 3 hours, with a 3-week interval between courses. _Single-agent second-line therapy after failure of combination chemotherapy for metastatic disease_: Paclitaxel 175 mg/m2 administered intravenously over 3 hours every 3 weeks. **Non-Small Cell Lung Carcinoma:** _Combination therapy_: For previously untreated patients, the recommended dosing regimen given with a 3-week interval between courses is, paclitaxel 175 mg/m2 administered intravenously over 3 hours followed by a platinum compound. Alternatively, a more myelosuppressive regimen of paclitaxel may be administered intravenously 135 mg/m2 over 24 hours followed by a platinum compound, with a 3-week interval between courses. _Single-agent therapy_: Paclitaxel 175 to 225 mg/m2 administered intravenously over 3 hours every 3 weeks. **AIDS-Related Kaposi’s Sarcoma:** _Second-line therapy_: Paclitaxel 135 mg/m2 administered intravenously over 3 hours with a 3-week interval between courses or 100mg/m2 administered intravenously over 3 hours with a 2-week interval between courses (dose intensity 45–50 mg/m2/week). Based upon the immunosuppression observed in patients with advanced HIV disease, the following modifications are recommended in these patients. 1. The dose of dexamethasone as one of the three premedication drugs should be reduced to 10 mg orally 2. Treatment with paclitaxel should be initiated or repeated only if the neutrophil count is at least 1000 cells/mm3 3. The dose of subsequent courses of paclitaxel should be reduced by 20% for those patients who experience severe neutropenia (<500 cells/mm3 for a week or longer) 4. Concomitant hematopoietic growth factor (G-CSF) should be initiated as clinically indicated. _Hepatic Impairment_ Patients with hepatic impairment may be at increased risk of toxicity, particularly grade III–IV myelosuppression. Dose adjustment is recommended, as shown in Table 1 for both 3- and 24-hour infusions. Patients should be monitored closely for the development of profound myelosuppression. (See **CLINICAL PHARMACOLOGY: Special Populations, Hepatic Impairment** section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) 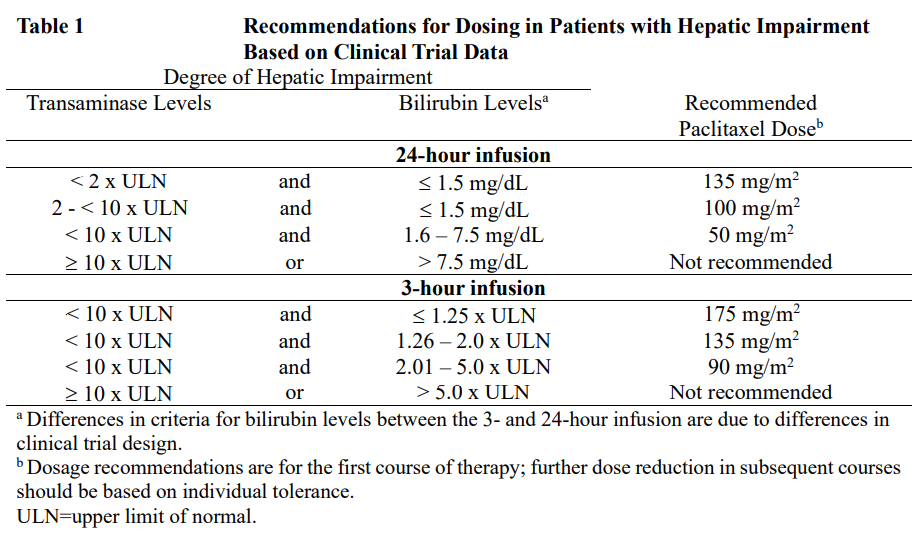 _Incompatibilities_ Contact of the undiluted concentrate with plasticized PVC equipment or devices used to prepare solutions for infusion is not recommended. In order to minimize patient exposure to the plasticizer DEHP \[di-(2-ethylhexyl)phthalate\], which may be leached from PVC infusion bags or sets, diluted paclitaxel solutions should be stored in bottles (glass, polypropylene) or plastic bags (polypropylene, polyolefin) and administered through polyethylene-lined administration sets. (See **Special Instruction for Use, Handling and Disposal** section.) _Special Instruction for Use, Handling and Disposal_ Paclitaxel is a cytotoxic anticancer drug and caution should be exercised in handling paclitaxel. The use of gloves is recommended. If paclitaxel solution contacts the skin, wash the skin immediately and thoroughly with soap and water. If paclitaxel contacts mucous membranes, the membranes should be flushed thoroughly with water. Following topical exposure, events have included tingling, burning and redness. Upon inhalation, dyspnea, chest pain, burning eyes, sore throat and nausea have been reported. Given the possibility of extravasation, it is advisable to closely monitor the infusion site for possible infiltration during drug administration (see **SPECIAL WARNINGS AND PRECAUTIONS FOR USE: Injection Site Reaction** section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Paclitaxel should be administered through an in-line filter with a microporous membrane not greater than 0.22 microns. Use of filter devices which incorporate short inlet and outlet PVC-coated tubing has not resulted in significant leaching of DEHP.** **Paclitaxel must be diluted prior to infusion to a final concentration of 0.3 to 1.2 mg/mL.** Paclitaxel should be diluted in one of the following: 0.9% Sodium Chloride Injection, 5% Dextrose Injection, 5% Dextrose and 0.9% Sodium Chloride Injection, or 5% Dextrose in Ringer's Injection. Upon preparation, solutions may show haziness, which is attributed to the formulation vehicle. No significant losses in potency have been noted following delivery of the solution through intravenous tubing containing an in-line 0.22 micron filter. Paclitaxel solutions should be prepared and stored in glass, polypropylene, or polyolefin containers. Non-PVC containing administration sets, such as those that are polyethylene-lined, should be used. (See **Incompatibilities** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) Diluted solutions are chemically and physically stable for up to 27 hours at temperature between 15°C to 30°C and room lighting conditions; infusions should be completed within this timeframe. There have been rare reports of precipitation with longer than the recommended 3-hour infusion schedules. Excessive agitation, vibration or shaking may induce precipitation and should be avoided. Infusion sets should be flushed thoroughly with a compatible diluent before use. Devices with spikes should not be used with vials of paclitaxel since they can cause the stopper to collapse resulting in loss of sterile integrity of the paclitaxel solution. Procedures for proper handling and disposal of anticancer drugs should be considered. To minimize the risk of dermal exposure, always wear impervious gloves when handling vials containing paclitaxel. This includes all handling activities in clinical settings, pharmacies, storerooms, and home healthcare settings, including during unpacking and inspection, transport within a facility, and dose preparation and administration.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Paclitaxel 6 mg/ml concentrate for solution for infusion is indicated for the treatment of the following: **Ovarian carcinoma:** - First-line therapy in a combination with platinum compound for the treatment of advanced metastatic carcinoma of the ovary. - Second-line therapy for the treatment of advanced metastatic carcinoma of the ovary. **Breast carcinoma:** - Adjuvant treatment of node-positive breast cancer administered sequentially to standard combination therapy. - First-line therapy of advanced or metastatic breast cancer after relapse within 6 months of adjuvant therapy. Prior therapy should have included an anthracycline unless clinically contraindicated. - First-line therapy of metastatic breast cancer in combination with trastuzumab in patients who over-express HER-2 as determined by immunohistochemistry. - First-line therapy of metastatic breast cancer in combination with an anthracycline in patients for whom anthracycline therapy is suitable. - Second-line therapy of advanced or metastatic breast cancer after failure of combination chemotherapy for metastatic disease. Prior therapy should have included an anthracycline unless clinically contraindicated. **Non-small cell lung carcinoma:** - First-line therapy in combination with a platinum compound or as a single agent for the treatment of non-small cell carcinoma of the lung in patients who are not candidates for potentially curative surgery and/or radiation therapy. **Kaposi's sarcoma:** - Second-line treatment of AIDS-related Kaposi’s Sarcoma.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_, especially Polyoxyethylated 35 castor oil (Macrogolglycerol ricinoleate 35) (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Lactation (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with baseline neutrophils < 1,500/mm3 (<1,000/mm3 for KS patients). In KS, patients with concurrent, serious, uncontrolled infections.
L01CD01
paclitaxel
Manufacturer Information
ADVAGEN PTE. LTD.
Sichuan Huiyu Pharmaceutical Co., Ltd
Active Ingredients
Documents
Package Inserts
Paclitaxel Advagen Concentrate for Solution for Infusion_PI.pdf
Approved: April 28, 2023
