Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**DOSAGE AND ADMINISTRATION** Voriconazole requires reconstitution of 10 mg/mL and subsequent dilution to 0.5 – 5mg/mL prior to administration as an infusion at a maximum rate of 3 mg/kg per hour over 1–3 hours. **Not for IV bolus injection** Electrolyte disturbance such as hypokalemia, hypomagnesemia and hypocalcemia should be corrected prior to initiation of Voriconazole therapy. **Dose for adults:** Therapy must be initiated with specified loading dose regimen of intravenous Voriconazole to achieve to plasma concentrations on Day 1 that are close to steady state. Voriconazole must not be infused concomitantly with any blood product or any short-term infusion of concentrated electrolytes, even if the two infusions are running in separate intravenous lines (or cannulas). Voriconazole can be infused at the same time as other intravenous solutions containing (non-concentrated) electrolytes, but must be infused through a separate line. Voriconazole can be infused at the same time as total parenteral nutrition, but must be infused in a separate line. If infused through a multiple-lumen catheter, TPN needs to be administered using a different port from the one used for voriconazole. Voriconazole must not be infused into the same line or cannula concomitantly with other intravenous products. 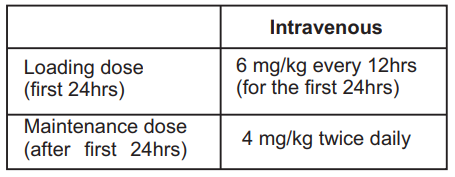 **Dose adjustments:** If patients are unable to tolerate treatment, reduce the intravenous maintenance dose to 3 mg/kg every 12 hours. Phenytoin may be co-administered with Voriconazole if the intravenous maintenance dose of Voriconazole is increased to 5 mg/ kg every 12 hours. Duration of therapy should be based on the severity of the patient's underlying disease recovery from immunosuppression, and clinical response. **Elderly:** No dose adjustment is necessary for elderly for patients. **Children:** Safely and effectiveness is pediatric patients below the age of 2 years has not been established therefore Voriconazole is not recommended for children less than 2 years of age. The recommended maintenance dosing regimen in pediatric patients 2 to <12 years is as follows. **Children aged 2 to <12 years:**  The pharmacokinetics and tolerability of higher doses have not been characterized in pediatric populations. If pediatric patients are unable to tolerate an intravenous dose of 7 mg/kg twice daily, a dose reduction from 7 mg/kg to 4 mg/kg twice daily may be considered based on the population pharmacokinetic analysis and previous clinical experience. This provides equivalent exposure to 3 mg/kg twice daily in adults. Use in pediatric patients aged 2 to <12 years with hepatic or renal insufficiency has not been studied. **Adolescents 12 to 16 years age:** should be given the same dose as adults. **Hepatic impairment:** No dose adjustment is necessary in patients with acute hepatic injury manifested by elevated liver function tests (ALAT, ASAT) but continued monitoring of liver function tests for further elevations is recommended. It is recommended that the Standard loading dose regimens be used but that the maintenance dose be halved in patients with mild to moderate hepatic cirrhosis (Child-Pugh class A and B). Voriconazole has not been studied in patients with severe hepatic cirrhosis (Child-Pugh class C) or in patients with chronic hepatitis B or chronic hepatitis C disease. Voriconazole has been associated with elevations in liver function tests and clinical signs of liver damage, such as jaundice, and should only be used in patients with severe hepatic insufficiency if the benefit outweighs the potential risk. Patients with hepatic insufficiency must be carefully monitored for drug toxicity. **Renal Impairment:** In patients with moderate or severe renal insufficiency (creatinine clearance <50 mL/min), Accumulation of the intravenous vehicle, SBECD occurs. Oral Voriconazole should not be administered to these patients, unless an assessment of the benefit/risk to the patient justifies the use of intravenous Voriconazole. Serum creatinine levels should be closely monitored in these patients and if increases occur, consideration should be given to changing to oral Voriconazole therapy. Voriconazole is hemodialyzed with clearance of 121 mL/min. The intravenous vehicle, SBECD is hemodialyzed with clearance of 55 mL/min. A 4 hour hemodialysis session does not remove a sufficient amount of Voriconazole to warrant dose adjustment. **Intravenous administration** **Reconstitution:** The powder is reconstituted with 19 mL of Water for Injection to obtain an extractable volume of 20 mL of clear concentrate containing 10 mg/mL of Voriconazole. It is recommended that a standard 20 mL (none automated) syringe be used to ensure that the exact amount (19.0 mL) of water for injection is dispensed. Discard the vial if a vacuum does not pull the diluent into the vial. Shake the vial until all the powder is dissolved. **Dilution:** The required volume of the 10 mg/mL Voriconazole concentrate should be further diluted as follows: 1. Calculate the volume of 10 mg/mL Voriconazole concentrate required based on the patient's weight (as per the table given below.) 2. In order to allow the required volume of Voriconazole to be added, withdraw and discard at least an equal volume of diluent from the infusion bag or bottle to be used. The volume of diluent remaining in the bag or bottle should be such that when the 10 mg/mL Voriconazole concentrate is added the final concentration is not less then 0.5 mg/mL or greater than 5 mg/mL. 3. Using a suitable size syringe and aseptic technique, withdraw the required volume of Voriconazole concentrate from the appropriate number of vials and add to the infusion bag or bottle. Discard partially used vials. The final Voriconazole solution must be infused over 1–3 hours at a maximum rate of 3 mg/kg per hour. **Required volumes of 10 mg/mL Voriconazole concentrate:**  Voriconazole is a single dose unpreserved sterile lyophile. Therefore, from a microbiological point of view, once reconstituted, the product should be used immediately. If not used immediately in use storage times and conditions prior to use are the responsibility of the user and should not be longer than 24 hours at 2° to 8° C (36° to 46° F). This medicinal product is for single use only and any unused solution should be discarded. Only clear solutions without particles should be used. The reconstituted solution can be diluted with 0.9% Sodium Chloride USP, Lactated Ringers USP, 5% Dextrose and Lactated Ringers USP, 5% Dextrose and 0.45% Sodium Chloride USP, 5% Dextrose USP, 5% Dextrose and 20mEq Potassium Chloride USP, 0.45% Sodium Chloride USP, 5% Dextrose and 0.9% Sodium Chloride USP. The compatibility of Voriconazole with diluents other than those described above is unknown. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Incompatibilities Voriconazole must not be infused into the same line or cannula concomitantly with other drug infusions including parenteral nutrition, e.g. Aminofusin 10% Plus. Aminofusin 10% Plus is physically incompatible with an increase in sub visible particulate matter after 24 hours storage at 4° C. Infusion of blood products must not occur simultaneously with Voriconazole even if the two infusions are running in separate intravenous lines (or cannulas). Infusion of total parenteral nutrition can occur simultaneously with Voriconazole, but must be infused through a separate line. Voriconazole must not be diluted with 4.2% Sodium Bicarbonate Infusion. The mildly alkaline nature of this diluent caused slight degradation of Voriconazole after 24 hours storage at room temperature. Although refrigerated storage is recommended following reconstitution, use of this diluent is not recommended as a precautionary measure. Compatibility with other concentration is unknown.
INTRAVENOUS
Medical Information
**THERAPEUTIC INDICATIONS** Voriconazole is indicated for use in treatment of the following conditions: - Treatment of invasive Aspergillosis - Treatment of fluconazole-resistant serious invasive candida infection (including _C.krusei_) - Serious fungal infection caused by _Scedosporium spp._ (Asexual form of Pseudallescheria body) and _Fusarium spp._,
**CONTRAINDICATIONS** Voriconazole is contraindicated in patients with known hypersensitivity to Voriconazole or to any of the excipients. Co-administration of CY3A4 substrates, terfenadine, astemizole, cisapride, pimozide or quinidine since increased plasma concentration of these drugs can lead to QTc prolongation and rare occurrences of torsades de points. Co-administration of Voriconazole with sirolimus is contraindicated because voriconazole significantly increases plasma concentrations of sirolimus. Co-administration of Voriconazole with rifampicin, rifampin, carbamazepine and long acting barbiturates (e.g. phenobarbital, mephobarbital) is contraindicated since these drugs are likely to decrease Plasma Voriconazole concentrations significantly. Co-administration of Voriconazole with ritonavir (400 mg twice daily) is contraindicated because ritonavir significantly decreases plasma voriconazole. Co-administration of Voriconazole with rifabutin is contraindicated since Voriconazole significantly increases rifabutin plasma concentration and nlabulin also significantly decreases voriconazole plasma concentrations. Co-administration of Voriconazole with ergot alkaloids (ergotamine and dihydroergotamine) is contraindicated because voriconazole may increase the plasma concentration of ergot alkaloids which may lead to ergotism. Co-administration of voriconazole with St. John's Wort is contraindicated. Co-administration of standard doses of voriconazole with efavirenz doses of 400 mg QD or higher is contraindicated because efavirenz significantly decreases plasma voriconazole concentrations in healthy subjects at these doses. Voriconazole also significantly increases efavirenz plasma concentrations (see drug interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Co-administration of voriconazole with naloxegol is contraindicated because voriconazole may significantly increase plasma concentrations of naloxegol which may precipitate opioid withdrawal symptoms (see drug interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Co-administration of voriconazole with tolvaptan is contraindicated because voriconazole may significantly increase plasma concentrations of tolvaptan (see drug interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Co-administration of voriconazole with venetoclax is contraindicated at initiation and during the venetoclax dose titration phase since voriconazole is likely to significantly increase plasma concentrations of venetoclax and increase risk of tumour lysis syndrome (see drug interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Co-administration of voriconazole with lurasidone is contraindicated since it may result in significant increases in lurasidone exposure and the potential for serious adverse reactions (see drug interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
J02AC03
voriconazole
Manufacturer Information
HETERO SINGAPORE PTE. LTD.
ASPIRO PHARMA LIMITED
Active Ingredients
Documents
Package Inserts
VORICA VORICONAZOLE POWDER FOR SOLUTION FOR INFUSION PI.pdf
Approved: May 4, 2023
