Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**POSOLOGY AND METHOD OF ADMINISTRATION** **Method of administration** Each tablet is to be taken orally either with or without food (see section PHARMACOKINETICS PROPERTIES – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). One tablet contains one treatment dose. The tablets should always be swallowed whole. The optimum daily dosage must be determined by careful titration of levodopa in each patient. The daily dose should preferably be optimised using one of the four available tablet strengths (50/12.5/200 mg, 100/25/200 mg, 150/37.5/200 mg or 200/50/200mg levodopa/ carbidopa/ entacapone). Patients should be instructed to take only one Stalevo tablet per dose administration. Patients receiving less than 70–100 mg carbidopa a day are more likely to experience nausea and vomiting. While the experience with total daily dosage greater than 200 mg carbidopa is limited, the maximum recommended daily dose of entacapone is 2000 mg and therefore the maximum Stalevo dose , for the Stalevo strengths of 50/12.5/200 mg, 100/25/200 mg and 150/37.5/200 mg, is 10 tablets per day. Ten (10) tablets of Stalevo 150/37.5/200 mg equals 375 mg of carbidopa a day. Therefore, using a maximum recommended daily dose of 375 mg of carbidopa, the maximum daily dose of Stalevo 200/50/200 mg is 7 tablets per day. The maximum total daily levodopa dose administered in the form of Stalevo should not exceed 1500 mg. **Starting Stalevo therapy** **Switching from levodopa/ DDC inhibitor (carbidopa or benserazide) preparations and entacapone to Stalevo** Usually Stalevo is intended for use in patients already receiving treatment with corresponding doses of standard-release levodopa/DDC inhibitor and entacapone. As with levodopa/carbidopa, non-selective monoamine oxidase (MAO) inhibitors are contraindicated for use with Stalevo. These inhibitors must be discontinued at least two weeks prior to initiating therapy with Stalevo. Stalevo may be administered concomitantly with the manufacturer's recommended dose of MAO inhibitors with selectivity for MAO type B (e.g., selegiline HCl). 1. Patients who are currently receiving treatment with entacapone and standard-release levodopa/carbidopa in doses equal to Stalevo tablet strengths can be directly switched to the corresponding Stalevo tablets, for example: 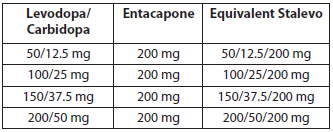 2. When initiating Stalevo therapy in patients currently receiving treatment with entacapone and levodopa/carbidopa in doses not equal to the available Stalevo tablet strengths (50/12.5/200 mg, 100/25/200 mg, 150/37.5/200 mg or 200/50/200mg), Stalevo dosing should be carefully titrated for optimal clinical response. At the start of therapy, Stalevo should be adjusted to correspond as closely as possible to the total daily dose of levodopa currently used. 3. When initiating Stalevo in patients currently treated with entacapone and levodopa/benserazide in a standard-release formulation, treatment should be stopped for one night and Stalevo therapy started the next morning. The therapy should begin with a dosage of Stalevo that will provide either the same amount of levodopa or slightly (5–10%) more. 4. There are no data on transferring patients from controlled-release formulations or standard release preparations with a 10:1 ratio of levodopa/DDC inhibitor to Stalevo. **Switching in patients not currently treated with entacapone to Stalevo** As with levodopa/carbidopa, non-selective monoamine oxidase (MAO) inhibitors are contraindicated for use with Stalevo. These inhibitors must be discontinued at least two weeks prior to initiating therapy with Stalevo. Stalevo may be administered concomitantly with the manufacturer's recommended dose of MAO inhibitors with selectivity for MAO type B (e.g., selegiline HCl). Initiation of Stalevo at a dosage corresponding to current treatment may be considered in some patients with Parkinson’s disease and end-of-dose motor fluctuations who are not stabilised on their current standard-release levodopa/DDC inhibitor treatment. However, a direct switch from levodopa/DDC inhibitor to Stalevo is not recommended for patients who have dyskinesias or whose daily levodopa dose is above 600 mg. In such patients it is advisable to introduce entacapone treatment as a separate medication (entacapone tablets) and adjust the levodopa dose if necessary, before switching to Stalevo. Entacapone enhances the effects of levodopa. It may therefore be necessary, particularly in patients with dyskinesia, to reduce levodopa dosage by 10–30% within the first days to first weeks after initiating Stalevo treatment. The daily dose of levodopa can be reduced by extending the dosing intervals and/or by reducing the amount of levodopa per dose, according to the clinical condition of the patient. **Dosage adjustment during the course of the treatment** When more levodopa is required, an increase in the frequency of doses and/or the use of an alternative strength of Stalevo should be considered, within the dosage recommendations. When less levodopa is required, the total daily dosage of Stalevo should be reduced either by decreasing the frequency of administration by extending the time between doses, or by decreasing the strength of Stalevo at an administration. If other levodopa products are used concomitantly with a Stalevo tablet, the maximum dosage recommendations should be followed. **Discontinuation of Stalevo therapy** If Stalevo treatment (levodopa/carbidopa/entacapone) is discontinued and the patient is switched to levodopa/DDC inhibitor therapy without entacapone, it is necessary to adjust the dosing of other antiparkinsonian treatments, especially levodopa, to achieve a sufficient level of control of the parkinsonian symptoms (see section SPECIAL WARNINGS AND PRECAUTIONS FOR USE, rhabdomyolysis – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Children and adolescents** The safety and efficacy of Stalevo in children aged below 18 years have not been established. No data are available. Stalevo is not recommended for use in children below age of 18. **Elderly** No adjustment of Stalevo dosage is necessary in elderly patients. **Renal impairment** Renal impairment does not affect the pharmacokinetics of entacapone. No specific studies are reported on the pharmacokinetics of levodopa and carbidopa in patients with renal impairment, and Stalevo should therefore be administered with caution in patients with severe renal impairment including those receiving dialysis therapy (see section PHARMACOKINETICS PROPERTIES – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**THERAPEUTIC INDICATIONS** Stalevo is indicated for the treatment of patients with Parkinson’s disease and end-of-dose motor fluctuations not stabilised on levodopa/dopa decarboxylase (DDC) inhibitor treatment.
**CONTRAINDICATIONS** - Known hypersensitivity to the active substances or to any of the excipients. - Liver impairment. - Narrow-angle glaucoma. - Pheochromocytoma. - Co-administration of a non-selective monoamine oxidase (MAO-A and MAO-B) inhibitor (e.g. phenelzine, tranylcypromine). - Co-administration use of a selective MAO-A inhibitor and a selective MAO-B inhibitor (see section INTERACTIONS WITH OTHER MEDICINAL PRODUCTS AND OTHER FORMS OF INTERACTIONS, other antiparkinsonian medicinal products – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). These inhibitors must be discontinued at least two weeks prior to initiating therapy with Stalevo. - A history of Neuroleptic Malignant Syndrome (NMS) and/or non-traumatic rhabdomyolysis. - Because levodopa may activate malignant melanoma, Stalevo should not be used in patients with suspicious, undiagnosed skin lesions or a history of melanoma.
Manufacturer Information
ORION PHARMA (SG) PTE. LTD.
Orion Corporation, Orion Pharma
Orion Corporation, Orion Pharma (Primary and Secondary Packager)
Active Ingredients
Documents
Package Inserts
Stalevo Film Coated Tablets PI.pdf
Approved: April 1, 2021
