Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INHALANT
**Dosage and Administration** Vaporisers specifically calibrated for use with Sevoflurane should be used so that the concentration delivered can be accurately controlled. MAC (minimum alveolar concentration) value for Sevoflurane decrease with age and with the addition of nitrous oxide. The table below indicates average MAC values for different age groups. 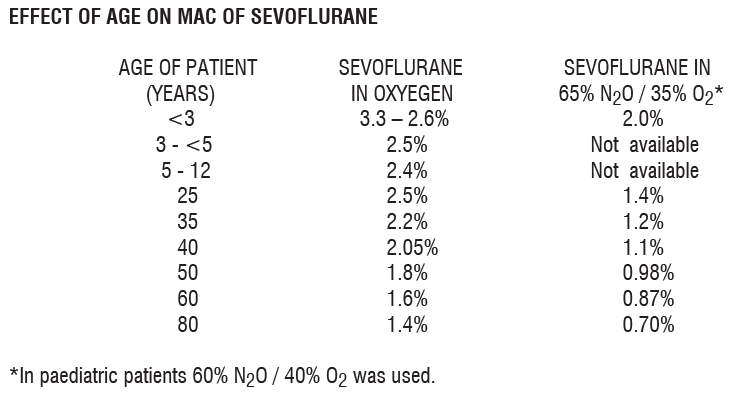 **Premedication:** Premedication should be selected according to the need of the individual patient, and at the discretion of the anaesthetist. The use of anticholinergic drugs is a matter of choice. **Induction:** Anaesthesia can be induced in adults and children with Sevoflurane. Dosage should be individualized and titrated to the desired effect according to the patients’s age and clinical status. A short acting barbiturate or other intravenous induction agent may be administered followed by inhalation of Sevoflurane. (See also “Interactions” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Induction with Sevoflurane may be achieved in oxygen or in combination with oxygen-nitrous oxide mixtures. In adults inspired concentrations of up to 5% Sevoflurane usually produce surgical anaesthesia in less than 2 minutes. In children, inspired concentrations of up to 7% Sevoflurane usually produce surgical anaesthesia in less than 2 minutes. Alternatively, for induction of anaesthesia in unpremedicated patients, inspired concentrations of up to 8% Sevoflurane may be used. **Maintenance:** Surgical levels of anaesthesia may be sustained with concentrations of 0.5 – 3% Sevoflurane with or without the concomitant use of nitrous oxide (see also “Drug Interactions” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Elderly:** As with other inhalation agents, lesser concentrations of Sevoflurane are normally required to maintain surgical anaesthesia. See above for MAC values. **Emergence:** Emergence times are generally short following Sevoflurane anaesthesia. Therefore, patients may require early post-operative pain relief.
RESPIRATORY (INHALATION)
Medical Information
**Indications** For induction and maintenance of general anaesthesia in-patient and out-patient surgery in both adults and children.
**Contraindications** Sevoflurane should not be used in patients with known or suspected sensitivity to Sevoflurane or to other halogenated inhalational anaesthetics (e.g. history of hepatotoxicity, usually including elevated liver enzymes, fever, leukocytosis and/or eosinophilia temporally related to anaesthesia with one of these agents). Sevoflurane is also contraindicated in patients known or suspected genetic susceptibility to malignant hyperthermia.
N01AB08
sevoflurane
Manufacturer Information
ALLIANCE PHARM PTE. LTD.
Piramal Critical Care Inc.
Active Ingredients
Documents
Package Inserts
Sojourn Inhalation Anaesthetic liquid PI.pdf
Approved: March 15, 2023
