Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2. Dosage and Administration** Posology **Nimenrix™** should be used in accordance with available official recommendations. 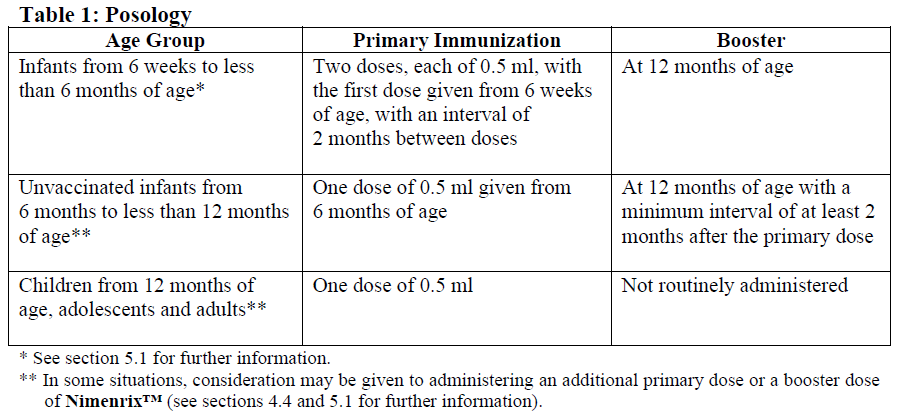 _Previously vaccinated children from 12 months of age, adolescents and adults_ **Nimenrix™** may be given as a booster dose to individuals who have previously received primary vaccination with a conjugated or plain polysaccharide meningococcal vaccine (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Special populations Individuals who have underlying conditions predisposing them to meningococcal infection due to anatomic or functional asplenia (such as sickle cell disease) may receive at least one dose of **Nimenrix™** (see sections 4.8 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of administration Immunization should be carried out by intramuscular injection only. In infants, the recommended injection site is the anterolateral aspect of the thigh. In individuals from 1 year of age, the recommended injection site is the anterolateral aspect of the thigh or the deltoid muscle (see sections 4.4 and 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For instructions on reconstitution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAMUSCULAR
Medical Information
**4.1. Indications** **Nimenrix™** is indicated for active immunization of individuals from 6 weeks of age against invasive meningococcal diseases caused by _Neisseria meningitidis_ groups A, C, W-135 and Y.
**4.3. Contraindications** **Nimenrix™** should not be administered to subjects with hypersensitivity to the active substances or to any of the excipients contained in the vaccine (see sections 2 and 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
J07AH08
meningococcus A,C,Y,W-135, tetravalent purified polysaccharides antigen conjugated
Manufacturer Information
PFIZER PRIVATE LIMITED
Catalent Belgium S.A. (diluent in pre-filled syringe)
Pfizer Manufacturing Belgium NV (diluent in pre-filled syringe)
Pfizer Manufacturing Belgium NV
GlaxoSmithKline Biologicals SA
Active Ingredients
Neisseria meningitidis group W-135 polysaccharide
5 micrograms
Neisseria meningitidis group Y polysaccharide
5 micrograms
Neisseria meningitidis group C polysaccharide
5 micrograms
Tetanus toxoid carrier protein
~44 micrograms
Documents
Package Inserts
Nimenrix Injection PI.pdf
Approved: July 7, 2022
