Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** Pharmaceutical Form: Oral Solution. Film-coated tablets. _EPIVIR_ therapy should be initiated by a physician experienced in the management of HIV infection. _EPIVIR_ can be taken with or without food. To ensure administration of the entire dose, the tablet(s) should ideally be swallowed without crushing. For patients who are unable to swallow tablets, lamivudine is available as an oral solution. Alternatively, the tablets may be crushed and added to a small amount of semi-solid food or liquid, all of which should be consumed immediately (see _Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **Adults, adolescents and children weighing at least 25 kg** The recommended dose of _EPIVIR_ is 300 mg daily. This may be administered as 150 mg (15 mL oral solution, 1 x 150 mg tablet) twice daily or 300 mg (30 mL oral solution, 2 x 150 mg tablets) once daily (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients changing to the once daily regimen should take 150 mg twice a day and switch to 300 mg once a day the following morning. When an evening once a day regimen is preferred, 150 mg of _EPIVIR_ should be taken on the first morning only, followed by 300 mg in the evening. When changing back to a twice daily regimen, patients should complete the day’s treatment and start 150 mg twice a day the following morning. - **Children weighing less than 25 kg** **Oral Solution** _Children from one year of age:_ The recommended dose is 0.5 mL/kg (5 mg/kg) twice daily or 1 mL/kg (10 mg/kg) once daily (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Children from three months to one year of age:_ The recommended dose is 0.5 mL/kg (5 mg/kg) twice daily. If a twice daily regimen is not feasible, a once daily regimen (10 mg/kg/day) could be considered. It should be taken into account that data for the once daily regimen are very limited in this population (see _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **150 mg Scored Tablets** Since an accurate dosing cannot be achieved with this formulation, dosing according to weight bands is recommended for _EPIVIR_ tablets. _Children weighing ≥ 20 kg to < 25 kg:_ The recommended dose is 225 mg daily. This may be administered as either 75 mg (one-half of a 150 mg tablet) taken in the morning and 150 mg (one whole 150 mg tablet) taken in the evening, or 225 mg (one and a half 150 mg tablets) taken once daily. _Children weighing 14 kg to < 20 kg:_ The recommended dose is 150 mg daily. This may be administered as 75 mg (one-half of a 150 mg tablet) taken twice daily, or 150 mg (one whole 150 mg tablet) taken once daily. Patients changing between lamivudine oral solution and lamivudine tablets should follow the dosing recommendations that are specific for the formulation (see _Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **Children less than three months of age** The limited data available are insufficient to propose specific dosage recommendations (see _Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **Renal impairment** Lamivudine plasma concentrations (AUC) are increased in patients with moderate to severe renal impairment due to decreased clearance (see _Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The dosage should therefore be reduced for patients with a creatinine clearance of < 30 mL/minute as shown in the table below. 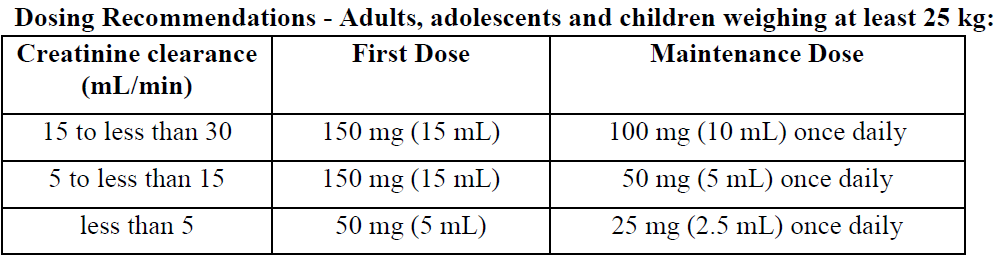 There are no data available on the use of lamivudine in children with renal impairment. A reduction in the dose and/or increase in the dosing interval should be considered in children aged at least three months and weighing less than 25 kg with a creatinine clearance of less than 50 mL/min. 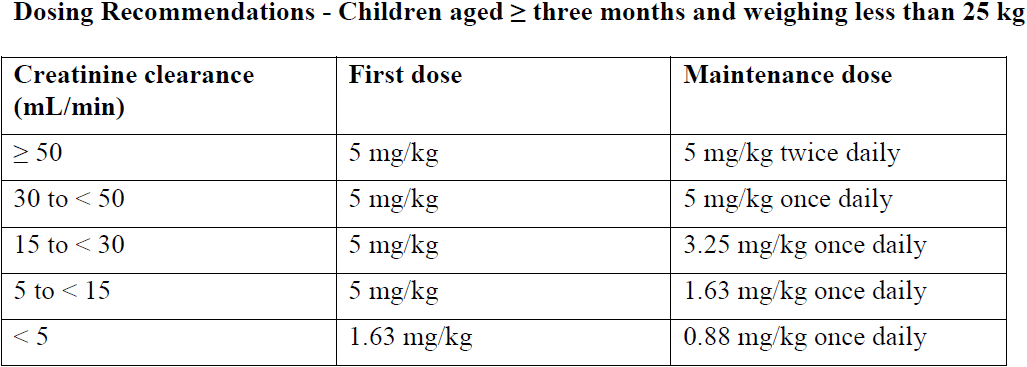 - **Hepatic impairment** No dose adjustment is necessary in patients with moderate or severe hepatic impairment unless accompanied by renal impairment (see _Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **Elderly** No specific data are available, however special care is advised in this age group due to age associated changes such as the decrease in renal function and alteration of haematological parameters.
ORAL
Medical Information
**Indications** _EPIVIR_, in combination with other antiretroviral agents, is indicated for the treatment of HIV - infected adults and children.
**Contraindications** The use of _EPIVIR_ is contraindicated in patients with known hypersensitivity to lamivudine or to any ingredient of the preparation.
J05AF05
lamivudine
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
GLAXO OPERATIONS UK LTD (TRADING AS GLAXO WELLCOME OPERATIONS)
Delpharm Poznan S.A.
Active Ingredients
Documents
Package Inserts
Epivir tablet and oral solution PI.pdf
Approved: February 22, 2023
