Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**2.2 Dosage and Method of Administration** Therapy with Recormon should be initiated by physicians experienced in the above mentioned indications. As anaphylactoid reactions were observed in isolated cases, it is recommended that the first dose be administered under medical supervision. Substitution by any other biological medicinal product requires the consent of the prescribing physician. The Recormon pre-filled syringe is ready for use. Only solutions which are clear or slightly opalescent, colourless and practically free of visible particles may be injected. Recormon in pre-filled syringe is a sterile but unpreserved product. Under no circumstances should more than one dose be administered per syringe. _Treatment of anemic patients with chronic renal failure_ The solution can be administered subcutaneously or intravenously. In case of intravenous administration, the solution should be injected over approximately 2 minutes, e.g. in hemodialysis patients via the arteriovenous fistula at the end of dialysis. For non-hemodialysed patients, subcutaneous administration should always be preferred in order to avoid puncture of peripheral veins. The recommended hemoglobin target is 10 – 12 g/dl. The target hemoglobin should be determined individually in the presence of hypertension or existing cardiovascular, cerebrovascular or peripheral vascular diseases. It is recommended that hemoglobin is monitored at regular intervals (e.g. every two to four weeks) until stabilised and periodically thereafter. Treatment with Recormon is divided into two stages: Correction phase - _Subcutaneous administration_: The initial dosage is 3 x 20 international units/kg body weight per week. The dosage may be increased every 4 weeks by 3 X 20 international units/kg body weight/week if the _Hb_ increase is not adequate ( _Hb < 1.5 g/L_ per week). The weekly dose can also be divided into daily doses. - _Intravenous administration_: The initial dosage is 3 x 40 international units/kg per week. The dosage may be raised after 4 weeks to 80 international units/kg - three times per week- and by further increments of 20 international units/kg if needed, three times per week, at monthly intervals. For both routes of administration, the maximum dose should not exceed 720 international units/kg per week. Maintenance phase To maintain hemoglobin between 10 – 12 g/dl, the dosage is initially reduced to half of the previously administered amount. Subsequently, the dose is adjusted at intervals of one or two weeks individually for the patient (maintenance dose). In the case of subcutaneous administration, the weekly dose can be given as one injection per week or in divided doses three or seven times per week. Patients who are stable on a once weekly dosing regimen may be switched to once every two weeks administration. In this case dose increases may be necessary. Results of pediatric clinical studies have shown that, on average, the younger the patients, the higher the Recormon doses required. Nevertheless, the recommended dosing schedule should be followed as the individual response cannot be predicted. Treatment with Recormon is normally a long-term therapy. It can however, be interrupted, if necessary, at any time. The dose for each patient should be adjusted so that the hemoglobin concentration does not exceed 12 g/dl. If the hemoglobin is increasing and approaching 12 g/dl, the dose should be reduced by approximately 25 – 50%. If the hemoglobin continues to increase, the dose should be temporarily withheld until the hemoglobin begins to decrease, at which point therapy should be reinitiated at a dose approximately 25 – 50% below the previous dose. If the hemoglobin increases by more than 1 g/dl in any 2 week period, the dose should be decreased by approximately 25 – 50%. _Treatment of symptomatic anemia in cancer patients:_ The solution is administered subcutaneously; the weekly dose can be given as one injection per week or in divided doses 3 to 7 doses times per week. The target hemoglobin concentration should be up to 12g/dl (7.45 mmol/l) and it should not be exceeded. The recommended initial dose is 30,000 international units per week (corresponding to approximately 450 international units/kg body weight per week, based on average weighted patient). Recormon treatment is indicated if the hemoglobin value is ≤ 11 g/dl (6.83 mmol/l) at the start of chemotherapy. The recommended initial dose is 450 international units/kg body weight per week. If, after 4 weeks of therapy, the hemoglobin value has increased by at least 1 g/dl (0.62 mmol/l), the current dose should be continued. If the hemoglobin value has not increased by at least 1 g/dl (0.62 mmol/l), a doubling of the weekly dose should be considered. If hemoglobin falls by more than 1 g/dl (0.62 mmol/l) in the first cycle of chemotherapy despite concomitant Recormon therapy, further therapy may not be effective. If, after 8 weeks of therapy, the hemoglobin value has not increased by at least 1 g/dl (0.62 mmol/l), response is unlikely and treatment should be discontinued. The therapy should be continued for up to 4 weeks after the end of chemotherapy. The maximum dose should not exceed 60,000 international units per week. Once the therapeutic objective for an individual patient has been achieved, the dose should be reduced by 25 to 50% in order to maintain hemoglobin at that level. If required, further dose reduction may be instituted to ensure that hemoglobin level does not exceed 12 g/dl. If the rise in hemoglobin is greater than 2 g/dl (1.3mmol/l) in 4 weeks, the dose should be reduced by 25 to 50%. It is recommended that hemoglobin is monitored at regular intervals (e.g. every two weeks) until stabilised and periodically thereafter. _Treatment for increasing the amount of autologous blood_ The solution is administered intravenously over approximately 2 minutes, or subcutaneously. Recormon is administered twice weekly over 4 weeks. On those occasions where the patient’s PCV allows blood donation, i.e. PCV ≥ 33% or Hb 11g/dl, Recormon is administered at the end of blood donation. The dosage must be determined by the surgical team individually for each patient as a function of the required amount of predonated blood and the endogenous red cell reserve: 1. The required amount of predonated blood depends on the anticipated blood loss, use, if any, of blood conserving procedures and the physical condition of the patient. This amount should be that quantity which is expected to be sufficient to avoid homologous blood transfusions. The required amount of predonated blood is expressed in units whereby one unit in the nomogram is equivalent to 180 ml red cells. 2. The ability to donate blood depends predominantly on the patient’s blood volume and baseline PCV. Both variables determine the endogenous red cell reserve, which can be calculated according to the following formula. Endogenous red cell reserve = blood volume \[ml\] x (PCV - 33) ÷ 100 Women: blood volume \[ml\] = 41 \[ml/kg\] x body weight \[kg\] + 1200 \[ml\] Men: blood volume \[ml\] = 44 \[ml/kg\] x body weight \[kg\] + 1600 \[ml\] (body weight ≥ 45 kg) The indication for Recormon treatment and, if given, the single dose should be determined from the required amount of predonated blood and the endogenous red cell reserve according to the following graphs. 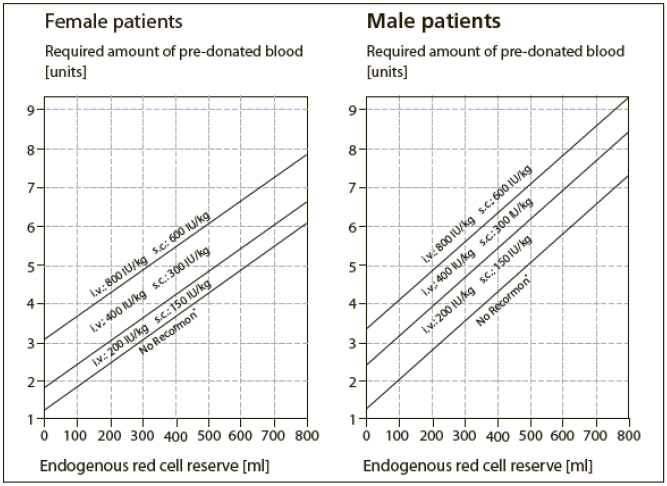 The single dose thus determined is administered twice weekly over 4 weeks. The maximum dose should not exceed 1600 international units/kg body weight per week for intravenous or 1200 international units/kg per week for subcutaneous administration.
INTRAVENOUS, SUBCUTANEOUS
Medical Information
**2.1 Therapeutic Indications** Recormon is indicated for: - Treatment of anemia associated with chronic renal failure (renal anemia) in patients on dialysis. - Treatment of symptomatic renal anemia in patients not yet undergoing dialysis. - Treatment of symptomatic anemia in adult patients with non-myeloid malignancies receiving chemotherapy. - Increasing the yield of autologous blood from patients in a predonation programme. Its use in this indication must be balanced against the reported increased risk of thromboembolic events. Treatment should only be given to patients with moderate anemia (Hb 10 to 13 g/dl \[6.2 to 8.1 mmol/l\], no iron deficiency) if blood conserving procedures are not available or insufficient when the scheduled major elective surgery requires a large volume of blood (4 or more units of blood for females; 5 or more units for males). \\* Deficiency is defined as an inappropriately low serum erythropoietin level in relation to the degree of anemia.
**2.3 Contraindications** Recormon must not be used in the presence of poorly controllable hypertension and known hypersensitivity to the active substance or to any of the excipients. In the indication “increasing the yield of autologous blood”, Recormon must not be used in patients who, in the month preceding treatment, have suffered a myocardial infarction or stroke, patients with unstable angina pectoris, or patients who are at risk of deep venous thrombosis such as those with a history of venous thromboembolic disease.
B03XA01
erythropoietin
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
ROCHE DIAGNOSTICS GMBH
Vetter Pharma Fertigung GmbH & Co KG
Active Ingredients
Documents
Package Inserts
Recormon Pre-filled Syringe PI.pdf
Approved: February 28, 2022
