Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CREAM
**2 DOSAGE AND ADMINISTRATION** **2.1 General Dosing Information** **PLIAGLIS Cream should only be applied to intact skin.** **For use in adults only.** - For superficial dermatological procedures such as dermal filler injection, non-ablative laser facial resurfacing, or pulsed-dye laser therapy, apply PLIAGLIS Cream to intact skin for 20–30 minutes prior to the procedure. See Table 1 for instructions on the amount to apply. - For superficial dermatological procedures such as laser-assisted tattoo removal, apply PLIAGLIS Cream to intact skin for 60 minutes prior to the procedure. See Table 1 for instructions on the amount to apply. The dose of PLIAGLIS Cream that provides effective local dermal analgesia depends on the duration of the application. Although not specifically studied, a shorter duration of application may result in a less complete dermal analgesia or a shorter duration of adequate dermal analgesia. **2.2 Dosage Information** **Determine the amount of drug to apply.** The amount (length) of PLIAGLIS Cream that should be dispensed is determined by the size of the area to be treated (see Table 1). 1. Squeeze out and measure the amount of PLIAGLIS Cream that approximates the amount required to achieve proper coverage. 2. Spread PLIAGLIS Cream evenly and thinly (approximately 1 mm or the thickness of a dime) across the treatment area using a flat-surfaced tool such as a metal spatula or tongue depressor. 3. After waiting the required application time, remove the PLIAGLIS Cream by grasping a free-edge with your fingers and pulling it away from the skin. 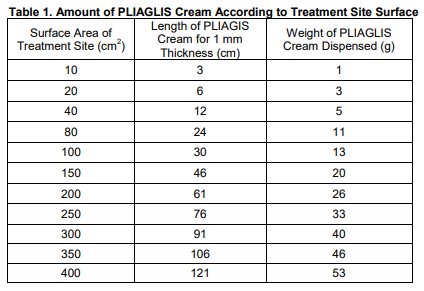 **2.3 Important Dosage and Administration Instructions** **Important Dosage and Administration instructions include:** - Remove PLIAGLIS Cream if skin irritation or a burning sensation occurs during application. - In order to minimize the risk of systemic toxicity, do not exceed the recommended amount of drug to apply or the duration of the application \[see Overdosage (10) – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. - Avoid eye contact with PLIAGLIS Cream. - Wash hands after handling PLIAGLIS Cream. - Upon removal from the treatment site, discard the used PLIAGLIS Cream in a location that is out of the reach of children and pets. Access to PLIAGLIS Cream by children or pets should be prevented during usage and storage of the product \[see WARNINGS and PRECAUTIONS (5.2) – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
CUTANEOUS
Medical Information
**1 INDICATIONS AND USAGE** PLIAGLIS Cream is a combination of lidocaine, an amide local anesthetic, and tetracaine, an ester local anesthetic, and is indicated for use on intact skin in adults to provide topical local analgesia for superficial dermatological procedures such as dermal filler injection, pulsed dye laser therapy, facial laser resurfacing, and laser-assisted tattoo removal.
**4 CONTRAINDICATIONS** - PLIAGLIS Cream is contraindicated in patients with a known history of sensitivity to lidocaine or tetracaine, local anesthetics of the amide or ester type, or to any other component of the product \[see Warnings and Precautions (5.4) – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. - PLIAGLIS Cream is contraindicated in patients with para-aminobenzoic acid (PABA) hypersensitivity.
N01BB52
lidocaine, combinations
Manufacturer Information
PHARMENG TECHNOLOGY PTE. LTD.
Laboratoires Galderma
Active Ingredients
Documents
Package Inserts
Pliaglis Cream PI.pdf
Approved: June 2, 2015
