Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Therapy with CABOMETYX should be initiated by a physician experienced in the administration of anticancer medicinal products. Posology _CABOMETYX as monotherapy_ For RCC, HCC and DTC, the recommended dose of CABOMETYX is 60 mg once daily. Treatment should continue until the patient is no longer clinically benefiting from therapy or until unacceptable toxicity occurs. _CABOMETYX in combination with nivolumab in first-line advanced RCC_ The recommended dose of CABOMETYX is 40 mg once daily in combination with nivolumab administered intravenously at either 240 mg every 2 weeks or 480 mg every 4 weeks. The treatment should continue until disease progression or unacceptable toxicity. Nivolumab should be continued until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression (see the Summary of Product Characteristics (SmPC) for posology of nivolumab). _Treatment modification_ Management of suspected adverse drug reactions may require temporary interruption and/or dose reduction (see Table 1). When dose reduction is necessary in monotherapy, it is recommended to reduce to 40 mg daily, and then to 20 mg daily. When CABOMETYX is administered in combination with nivolumab, it is recommended to reduce the dose to 20 mg of CABOMETYX once daily, and then to 20 mg every other day (refer to the nivolumab SmPC for recommended treatment modification for nivolumab). Dose interruptions are recommended for management of CTCAE Grade 3 or greater toxicities or intolerable Grade 2 toxicities. Dose reductions are recommended for events that, if persistent, could become serious or intolerable. If a patient misses a dose, the missed dose should not be taken if it is less than 12 hours before the next dose. 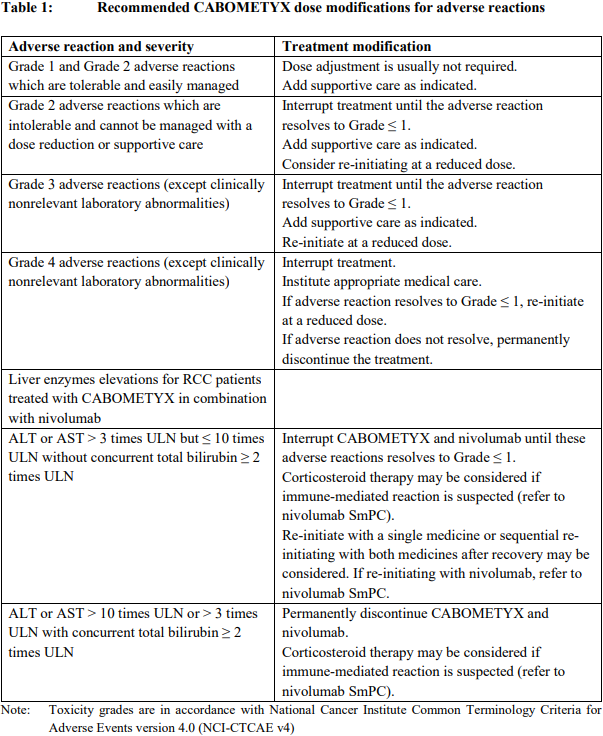 _Concomitant medicinal products_ Concomitant medicinal products that are strong inhibitors of CYP3A4 should be used with caution, and chronic use of concomitant medicinal products that are strong inducers of CYP3A4 should be avoided (see sections 4.4 and 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Selection of an alternative concomitant medicinal product with no or minimal potential to induce or inhibit CYP3A4 should be considered. Special populations _Elderly_ No specific dose adjustment for the use of cabozantinib in elderly patients (≥ 65 years) is recommended. _Race_ No dose adjustment is necessary based on ethnicity (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ Cabozantinib should be used with caution in patients with mild or moderate renal impairment. Cabozantinib is not recommended for use in patients with severe renal impairment as safety and efficacy have not been established in this population. _Hepatic impairment_ In patients with mild hepatic impairment, no dose adjustment is required. Since only limited data are available for patients with moderate hepatic impairment (Child Pugh B), no dosing recommendation can be provided. Close monitoring of overall safety is recommended in these patients (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There is no clinical experience in patients with severe hepatic impairment (Child Pugh C), so cabozantinib is not recommended for use in these patients (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Cardiac impairment_ There is limited data in patients with cardiac impairment. No specific dosing recommendations can be made. _Paediatric population_ The safety and efficacy of cabozantinib in children and adolescents aged < 18 years have not yet been established. Currently available data are described in section 5.2 but no recommendation on a posology can be made – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Method of administration CABOMETYX is for oral use. The tablets should be swallowed whole and not crushed. Patients should be instructed to not eat anything for at least 2 hours before through 1 hour after taking CABOMETYX.
ORAL
Medical Information
**4.1 Therapeutic indications** Renal cell carcinoma (RCC) CABOMETYX is indicated as monotherapy for advanced renal cell carcinoma - as first-line treatment of adult patients with intermediate or poor risk (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), - in adults following prior vascular endothelial growth factor (VEGF)-targeted therapy (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). CABOMETYX, in combination with nivolumab, is indicated for the first-line treatment of advanced renal cell carcinoma in adults (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Hepatocellular carcinoma (HCC) CABOMETYX is indicated as monotherapy for the treatment of hepatocellular carcinoma in adults who have previously been treated with sorafenib. Differentiated thyroid carcinoma (DTC) CABOMETYX is indicated as monotherapy for the treatment of adult patients with locally advanced or metastatic differentiated thyroid carcinoma (DTC), refractory or not eligible to radioactive iodine (RAI) who have progressed during or after prior systemic therapy.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01EX07
cabozantinib
Manufacturer Information
IPSEN PHARMA SINGAPORE PTE. LTD.
Patheon Inc.
Active Ingredients
Documents
Package Inserts
Cabometyx PI.pdf
Approved: January 5, 2023
