Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
_**Posology and method of administration**_ Treatment should only be initiated and monitored by a doctor with experience in the treatment of pulmonary arterial hypertension and systemic sclerosis. Tracleer® should be taken orally in the morning and evening, with or without food. The film-coated tablets are to be swallowed with water. Patients must be urged to refrain from swallowing the desiccant in the white high density polyethylene bottle. _Pulmonary arterial hypertension (PAH)_ Treatment with Tracleer® should be started at a dosage of twice daily 62.5 mg over a period of four weeks, followed by an increase to a maintenance dose of twice daily 125 mg. _Systemic sclerosis with active digital ulcer disease_ Treatment with Tracleer® should be started at a dosage of 62.5 mg twice daily over a period of four weeks, followed by an increase to a maintenance dose of 125 mg twice daily. Experience from controlled clinical trials in this indication is limited to a period of 6 months. Response to treatment and the necessity of continuing treatment should be regularly re-evaluated. _Discontinuation of treatment_ There has been no adequate experience of sudden discontinuation of Tracleer® treatment. There is no evidence of an acute rebound effect. To avoid possible severe clinical deterioration from any rebound effect, the possibility should be considered of gradually reducing the dose, by halving it for 3 to 7 days. It is recommended that the patient should be closely monitored during the discontinuation. _Dosage of patients with liver disorders_ No dose adjustment is necessary for patients with mildly impaired liver function (i.e. Child-Pugh class A) (see section on «Pharmacokinetic properties» – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Tracleer® is contraindicated in patients with moderate to severe liver disorders or where liver aminotransferase values are more than three times the upper limit of normal prior to the start of treatment (see sections on «Contraindications», «Special warnings and precautions for use» and «Pharmacokinetic properties» – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). See section on «Special warnings and precautions for use» – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ for course of action if liver aminotransferase values rise during treatment. _Dosage in patients with kidney disorders_ No dose adjustment is needed in patients with impaired renal function or in patients on dialysis (see section on «Pharmacokinetic properties» – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Dosage in older patients_ There have been no adequate studies on the affect of age. _Use in children and adolescents_ - Pulmonary arterial hypertension There have been no adequate studies on efficacy and safety in patients aged under 12 years. The following dosage scheme was used in trial AC-052-356 (BREATHE-3): 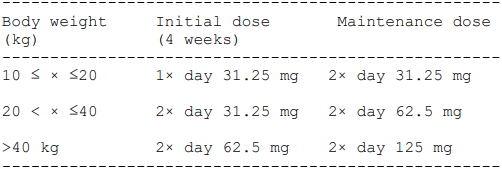 \\* The 31.25 mg dose was obtained from the not divisible 62.5 mg film-coated tablet by using a tablet splitter. There are, however, no data on the content of active substance in the halved tablets. The trial was designed to determine the pharmacokinetics in children. However, the number of patients in each dosage group was too low to establish the optimal dosage regimen for patients aged under 12 years. The pharmacokinetic results showed that the systemic bioavailability was lower than in adults with pulmonary hypertension, which might lead to suboptimal activity on the pulmonary vascular system. There are currently no adequate data on changes in haemodynamics in children. There are no data for children aged under 3 years. There are only limited data for patients weighing under 40 kg. For dosage for these patients, please refer to the dosage table in the section on «Use in children and adolescents». The 31.25 mg dose was obtained from the unscored 62.5 mg film-coated tablet by using a tablet splitter. There are however no data on the content of active substance in the halved tablets. - Systemic sclerosis with active digital ulcer disease There are no data on the safety and efficacy in patients aged under 18 years.
ORAL
Medical Information
_**Indications / Areas of use**_ Treatment of pulmonary arterial hypertension (PAH) in patients with WHO functional class III or IV symptoms, to improve exercise ability and decrease the rate of clinical worsening. Some improvements have also been shown in patients with PAH WHO functional class II. Reduction in the number of new digital ulcers in patients with systemic sclerosis with active digital ulcer disease.
_**Contraindications**_ Hypersensitivity to bosentan or to one of the excipients. Moderate to severe liver disorders (Child-Pugh class B or C see section on «Pharmacokinetic properties» – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Raised liver aminotransferases before the start of treatment, i.e. aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) to more than three times the upper normal limit (see section on «Special warnings and precautions for use» – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Pregnancy. Women of child-bearing age who are not using adequate contraception. Simultaneous use of cyclosporine A and glibenclamide.
C02KX01
bosentan
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
PATHEON INC
Active Ingredients
Documents
Package Inserts
Tracleer Tablet PI.pdf
Approved: May 5, 2022
