Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION
**DOSAGE AND ADMINISTRATION** _**Folotyn**_ ® should be administered under the supervision of a qualified physician experienced in the use of antineoplastic agents. Appropriate management of complications is possible only when adequate diagnostic and treatment facilities are readily available. **Peripheral T-cell Lymphoma** The recommended dose of _**Folotyn**_ ® is 30 mg/m2 administered as an intravenous (IV) push over 3–5 minutes via the side port of a free-flowing 0.9% Sodium Chloride Injection, USP IV line once weekly for 6 weeks in 7-week cycles until progressive disease or unacceptable toxicity. For patients with severe renal impairment (eGFR 15 to < 30 mL/min/1.73 m2), the recommended dose of _**Folotyn**_ ® is 15 mg/m2. **Pretreatment Vitamin Supplementation** _Folic Acid_ Instruct patients to take 1.0 to 1.25 mg orally once daily beginning 10 days before the first dose of _**Folotyn**_ ®. Continue folic acid during treatment with _**Folotyn**_ ® and for 30 days after the last dose of _**Folotyn**_ ®. \[ _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. _Vitamin B12_ Administer vitamin B12 (1 mg) intramuscular injection within 10 weeks prior to the first dose of _**Folotyn**_ ® and every 8–10 weeks thereafter. Subsequent vitamin B12 injections may be given the same day as treatment with _**Folotyn**_ ® \[ _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. **Preparation and Administration Precautions** _**Folotyn**_ ® is a cytotoxic anticancer agent. Caution should be exercised in handling, preparing, and administering of the solution. The use of gloves and other protective clothing is recommended. If _**Folotyn**_ ® comes in contact with the skin, immediately and thoroughly wash with soap and water. If _**Folotyn**_ ® comes in contact with mucous membranes, flush thoroughly with water. **Preparation for Intravenous Push Administration** 1. _**Folotyn**_ ® vials should be refrigerated at 2–8°C (36–46°F) until use. 2. _**Folotyn**_ ® vials should be stored in original carton to protect from light until use. 3. _**Folotyn**_ ® is a clear, yellow solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use any vials exhibiting particulate matter or discoloration. 4. The calculated dose of _**Folotyn**_ ® should be aseptically withdrawn into a syringe for immediate use. 5. Do not dilute _**Folotyn**_ ®. 6. _**Folotyn**_ ® vials contain no preservatives and are intended for single use only. After withdrawal of dose, discard vial including any unused portion. 7. Unopened vial(s) of _**Folotyn**_ ® are stable if stored in the original carton at room temperature for 72 hours. Any vials left at room temperature for greater than 72 hours should be discarded. **Monitoring and Dose Modifications** Management of severe or intolerable adverse reactions may require dose omission, reduction, or interruption of _**Folotyn**_ ® therapy. _**Monitoring**_ Complete blood cell counts and severity of mucositis should be monitored weekly. Serum chemistry tests, including renal and hepatic function, should be performed prior to the start of the first and fourth dose of a given cycle. _**Dose Modification Recommendations**_ Prior to administering any dose of: - Mucositis should be ≤ Grade 1. - Platelet count should be ≥ 100,000/microlitre for first dose and ≥ 50,000/microlitre for all subsequent doses. - Absolute neutrophil count (ANC) should be ≥ 1,000/microlitre. Doses may be omitted or reduced based on patient tolerance. Omitted doses will not be made up at the end of the cycle; once a dose reduction occurs for toxicity, do not re-escalate. For dose modifications and omissions, use the guidelines in Tables 1, 2, and 3. For patients with severe renal impairment (eGFR 15 to < 30 mL/min/1.73 m2), the recommended starting dose of _**Folotyn**_ ® is 15 mg/m2 with dose modification to 10 mg/m2 for the toxicities specified in Tables 1, 2 and 3. 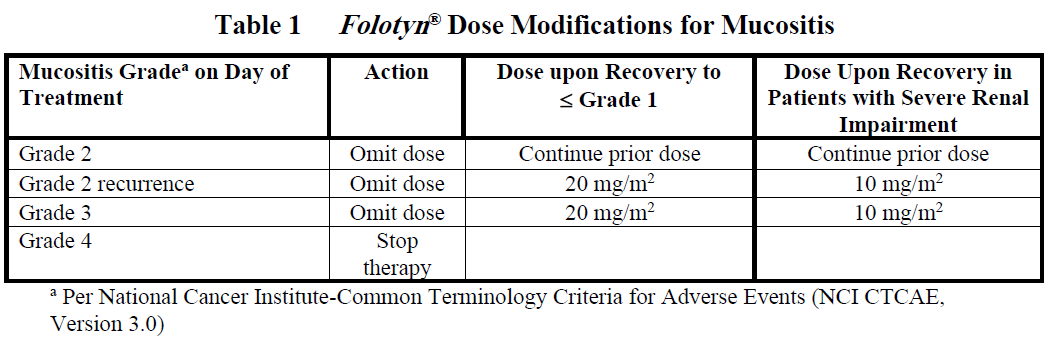 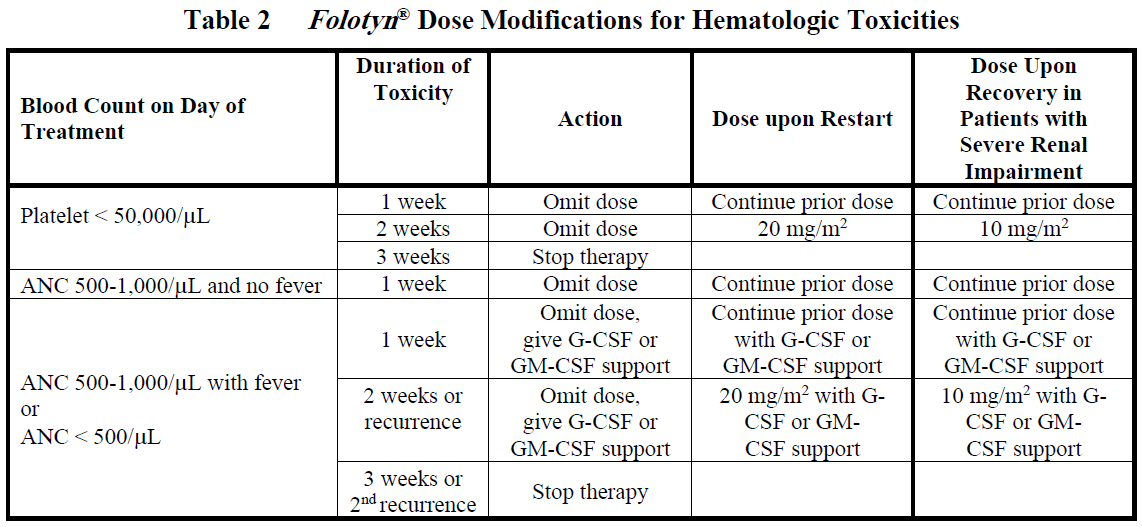 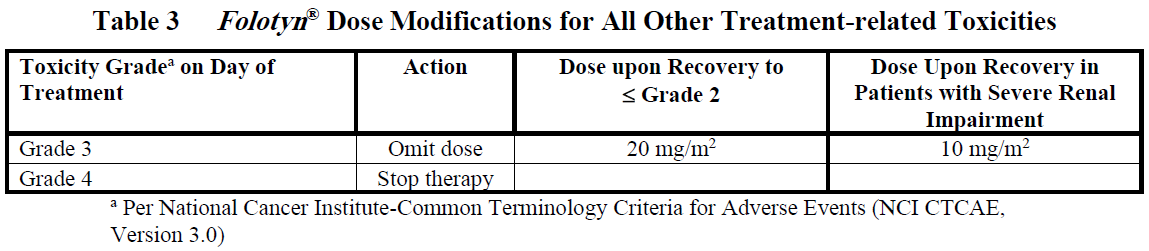
INTRAVENOUS
Medical Information
**INDICATIONS AND USAGE** _**Folotyn**_ ® is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). This indication is based on overall response rate with the view to induce responses sufficient to allow patients to be eligible for stem cell transplant. Clinical benefit such as improvement in progression-free survival or overall survival has not been demonstrated. In the pivotal open-label, single-arm, Phase II study, recruited patients were pre-treated with a median of 3 prior systemic therapies.
**CONTRAINDICATIONS** **None.**
L01BA05
pralatrexate
Manufacturer Information
MUNDIPHARMA PHARMACEUTICALS PTE. LTD.
Baxter Oncology GmbH
Active Ingredients
Documents
Package Inserts
FOLOTYN SOLUTION FOR INFUSION PI.pdf
Approved: January 31, 2022
