Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
AEROSOL, METERED
**DOSAGE, METHOD AND FREQUENCY OF ADMINISTRATION** The dosage depends on the type and severity of disease. Treatment is reserved for adults. Use of Atimos in children and adolescent is not recommended. **Asthma** Adults _**Maintenance therapy:**_ one or two puffs of 12 mcg (12–24 mcg) twice a day. The maximum recommended maintenance dose is 48mcg per day. If necessary, one or two additional puffs can be administered during the day to give relief from symptoms, provided the recommended daily maximum dose of 48mcg per day is not exceeded. However, if the need to take additional puffs becomes more frequent (for example more than two days a week) then a medical opinion should be sought to re-evaluate the therapy, because it could signify that the base illness is worsening. Atimos should not be used to relieve the acute symptoms of an asthma attack. In the event of an attack, short acting β2-agonist should be used. **Prophylaxis of bronchospasm induced by inhaled allergens, cold air, or exercise** Adults One 12 mcg puff should be inhaled approximately 15 minutes prior to exercise or exposure. Patients with severe asthma may need two 12 mcg puffs. **Instructions for use** Clinical efficacy and safety of Atimos inhaler in use with a spacer is not known, monitoring of condition is advised when product is use together with a spacer. The successful result of the treatment depends on a correct use of the inhaler. Inhaler test: to test the inhaler prior to using it for the first time (or if it has not been used for three days or more), remove the mouthpiece cover by carefully pressing the sides and then release one puff into the air to make sure that it works. The following instructions should be carefully followed: 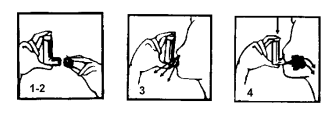 1)–2) Hold the inhaler between the thumb and forefinger, with the mouthpiece facing downwards, as shown in the figure. Remove the mouthpiece cover. 3) Breathe out completely, then place the mouthpiece in your mouth and close your lips around it; 4) While breathing in through your mouth, press once with the forefinger. Once the inspiration is complete, hold your breath for as long as possible. When finished, replace the mouthpiece cover. The mouthpiece should be kept clean by washing with warm water, after having removed the pressurised canister.
RESPIRATORY (INHALATION)
Medical Information
**THERAPEUTIC INDICATIONS** 1. Prophylaxis and treatment of bronchoconstriction in patients with asthma as an add-on to inhaled glucocorticosteroid treatment. 2. Prophylaxis of bronchospasm induced by inhaled allergens, cold air, or exercise.
**CONTRAINDICATIONS** Hypersensitivity to the active ingredient, or to any of the excipients, or any other chemically related substances. Tachyarrhythmiae, 3rd grade atrioventricular block, idiopathic hypertrophic subaortal stenosis, hypertrophic obstructive cardiomyopathy, idiopathic or drug-induced long-QT syndrome (QTc > 0.44 seconds), severe hyperthyroidism. Generally contraindicated during pregnancy and lactation (see also “Special Warnings” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
R03AC13
formoterol
Manufacturer Information
HYPHENS PHARMA PTE. LTD.
Chiesi Farmaceutici S.p.A.
Active Ingredients
Documents
Package Inserts
ATIMOS_PI 2 Feb 2010.pdf
Approved: February 3, 2010
