Basic Information
SYMBICORT TURBUHALER 160/4.5 mcg/dose
POWDER, METERED
Regulatory Information
SIN11681P
September 24, 2001
Prescription Only
Therapeutic
RESPIRATORY (INHALATION)
August 10, 2023
May 30, 2025
XR03AK07
Company Information
Active Ingredients
BUDESONIDE
Strength: 160 mcg/dose
FORMOTEROL FUMARATE DIHYDRATE
Strength: 4.5 mcg/dose
Detailed Information
Contraindications
**4.3 Contraindications** Hypersensitivity (allergy) to budesonide, formoterol or lactose (which contains small amounts of milk proteins).
Indication Information
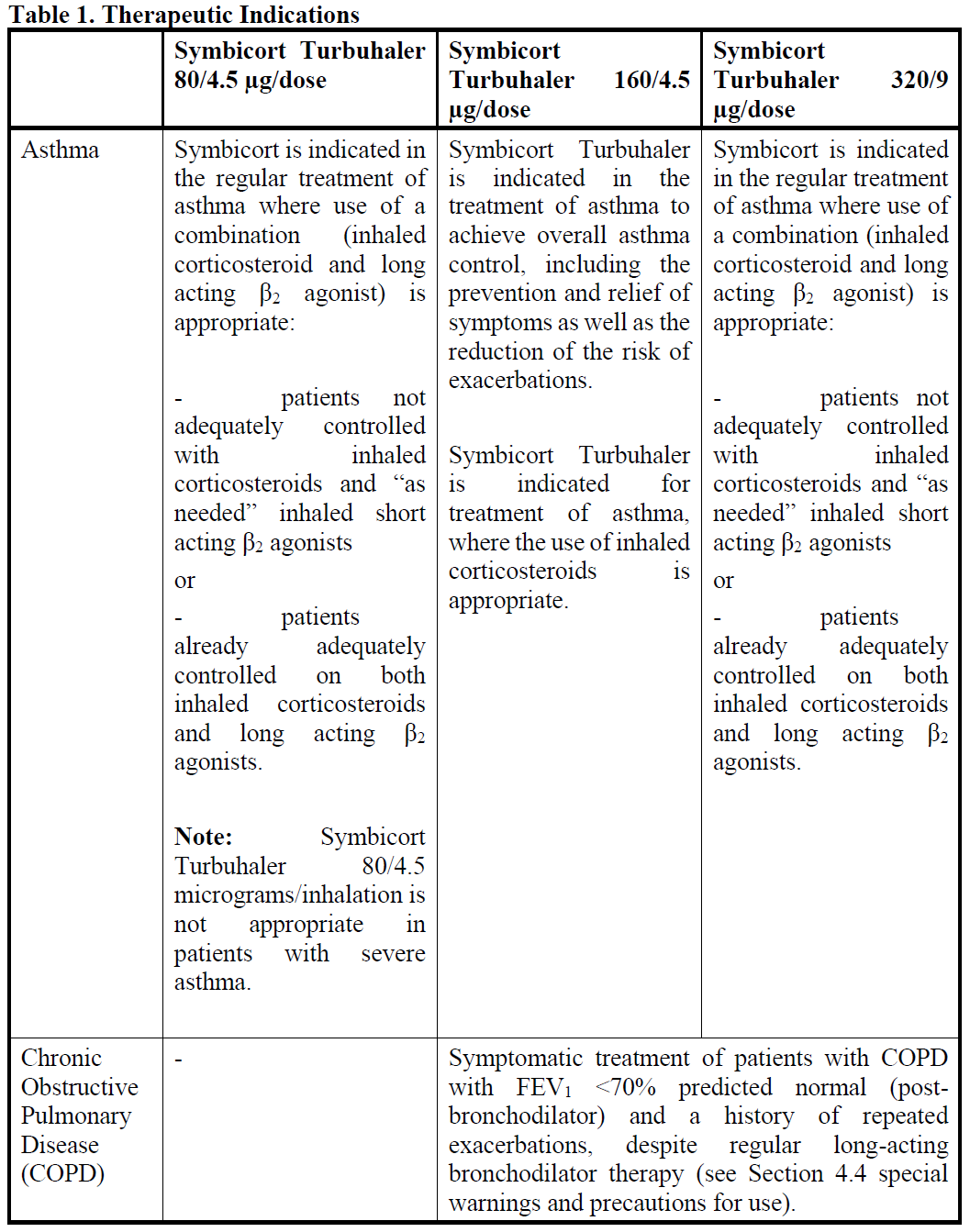
**4.1 Therapeutic indications**