Vitamins A, C, D and Fluoride Drops
Vitamins A, C, D and Fluoride Drops
d45644e1-f9ed-48b9-8baa-96e5a1560871
DIETARY SUPPLEMENT
Sep 16, 2025
H2-Pharma, LLC
DUNS: 028473634
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
VITAMIN A PALMITATE, ASCORBIC ACID, CHOLECALCIFEROL, and SODIUM FLUORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
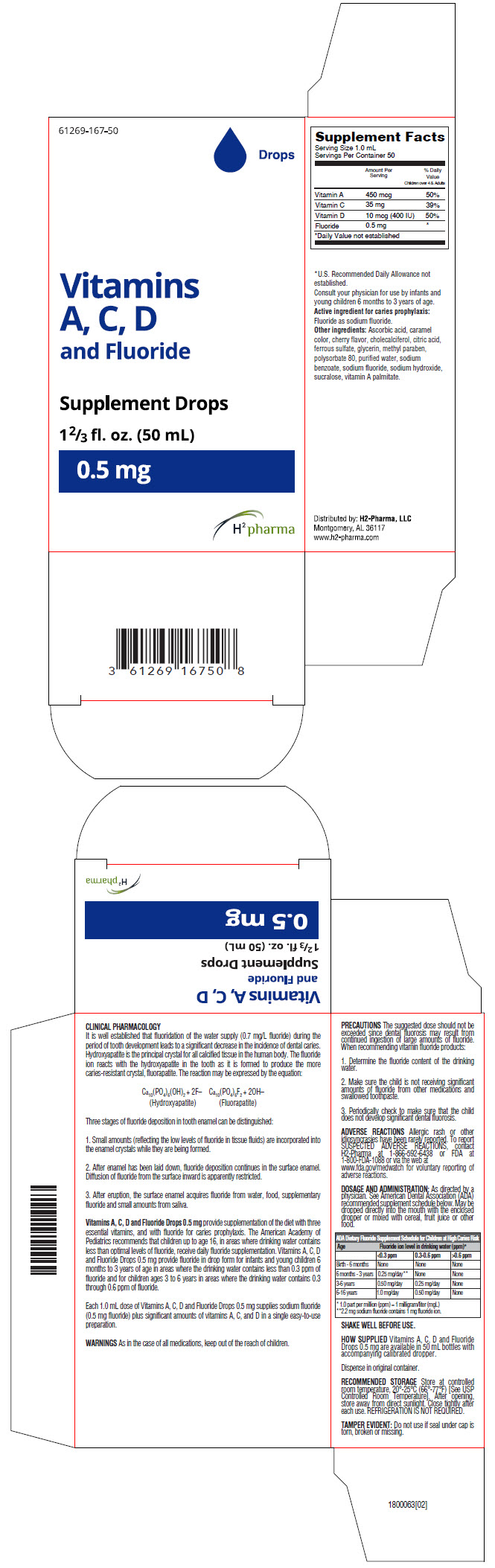
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Carton
61269-167-50
Drops
Vitamins
A, C, D
and Fluoride
Supplement Drops
1⅔ fl. oz. (50 mL)
0.5 mg
H2 pharma
STATEMENT OF IDENTITY SECTION
|
Supplement Facts | ||
|---|---|---|
|
Serving Size 1.0 mL | ||
|
Servings Per Container 50 | ||
|
Amount Per Serving |
% Daily Value | |
|
Children over 4 & Adults | ||
| ||
|
Vitamin A |
450 mcg |
50% |
|
Vitamin C |
35 mg |
39% |
|
Vitamin D |
10 mcg (400 IU) |
50% |
|
Fluoride |
0.5 mg |
|
*U.S. Recommended Daily Allowance not established.
Consult your physician for use by infants and young children 6 months to 3 years of age.
Active ingredient for caries prophylaxis: Fluoride as sodium fluoride.
Other ingredients: Ascorbic acid, caramel color, cherry flavor, cholecalciferol, citric acid, ferrous sulfate, glycerin, methyl paraben, polysorbate 80, purified water, sodium benzoate, sodium fluoride, sodium hydroxide, sucralose, vitamin A palmitate.
HEALTH CLAIM SECTION
Distributed by:H2-Pharma, LLC
Montgomery, AL 36117
WARNINGS SECTION
WARNINGS
As in the case of all medications, keep out of the reach of children.
PRECAUTIONS SECTION
PRECAUTIONS
The suggested dose should not be exceeded since dental fluorosis may result from continued ingestion of large amounts of fluoride. When recommending vitamin fluoride products:
- Determine the fluoride content of the drinking water.
- Make sure the child is not receiving significant amounts of fluoride from other medications and swallowed toothpaste.
- Periodically check to make sure that the child does not develop significant dental fluorosis.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
As directed by a physician. See American Dental Association (ADA) recommended supplement schedule below. May be dropped directly into the mouth with the enclosed dropper or mixed with cereal, fruit juice or other food.
ADA Dietary Fluoride Supplement Schedule for Children at High Caries Risk|
Age |
Fluoride ion level in drinking water (ppm)* | ||
|---|---|---|---|
|
<0.3 ppm |
0.3-0.6 ppm |
| |
| |||
|
Birth - 6 months |
None |
None |
None |
|
6 months - 3 years |
0.25 mg/day† |
None |
None |
|
3-6 years |
0.50 mg/day |
0.25 mg/day |
None |
|
6-16 years |
1.0 mg/day |
0.50 mg/day |
None |
SHAKE WELL BEFORE USE.
