Floriva
Floriva Chewable Fluoride Multivitamin
3d5fbe3a-a95b-4688-a96e-b34e6e1e868a
HUMAN PRESCRIPTION DRUG LABEL
Feb 1, 2024
BonGeo Pharmaceuticals, Inc.
DUNS: 964822022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
VITAMIN A ACETATE, .BETA.-CAROTENE, ASCORBIC ACID, CHOLECALCIFEROL, .ALPHA.-TOCOPHEROL, THIAMINE, RIBOFLAVIN, NIACINAMIDE, PYRIDOXINE, LEVOMEFOLATE GLUCOSAMINE, FOLIC ACID, CYANOCOBALAMIN, BIOTIN, ZINC GLUCONATE, CUPRIC OXIDE, and SODIUM FLUORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (21)
Drug Labeling Information
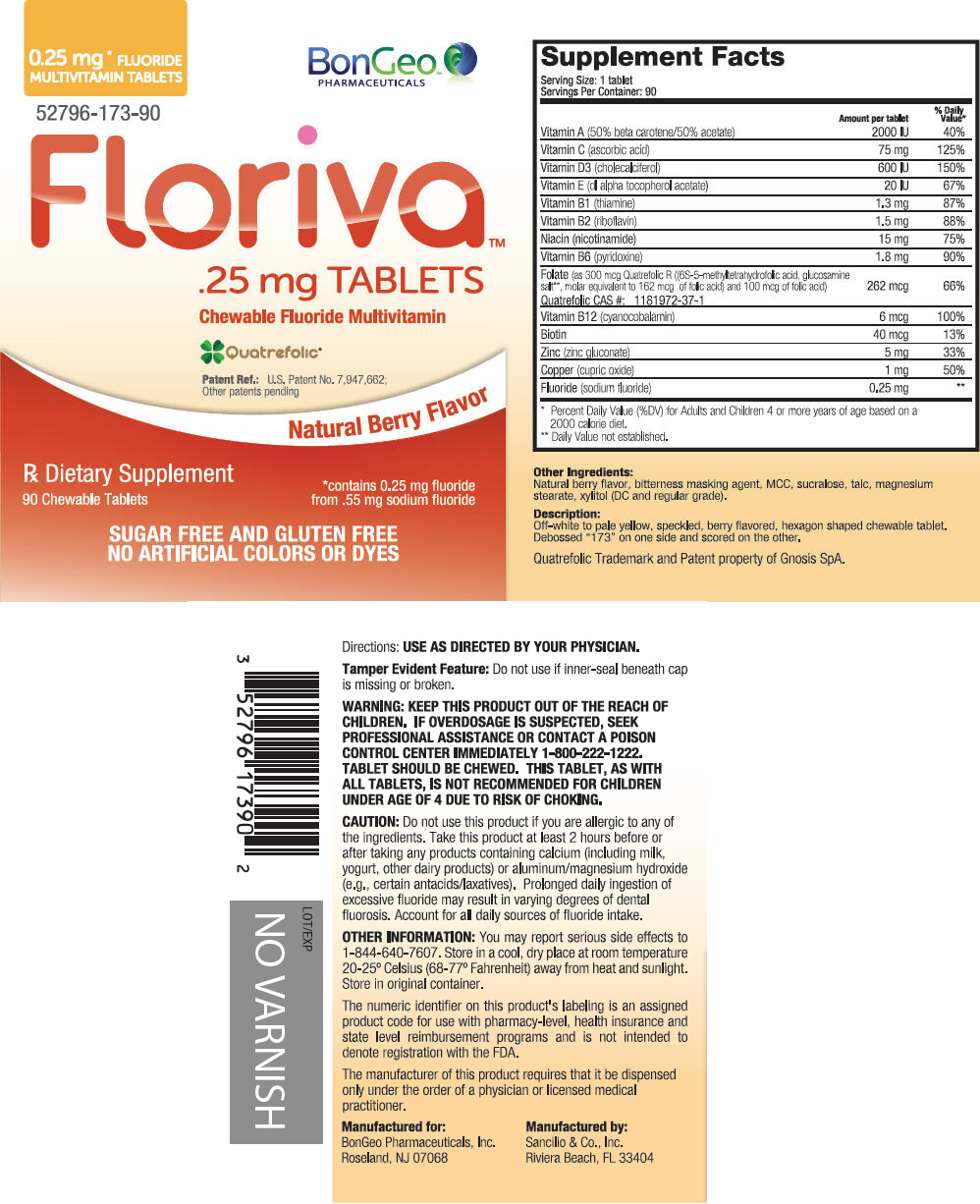
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle Label
0.25 mg FLUORIDE*
MULTIVITAMIN TABLETS
BonGeo™
PHARMACEUTICALS
52796-173-90
Floriva**™**
.25 mg TABLETS
Chewable Fluoride Multivitamin
Quatrefolic®
Patent Ref.: U.S. Patent No. 7,947,662;
Other patents pending
Natural Berry Flavor
Rx Dietary Supplement
90 Chewable Tablets
*contains 0.25 mg fluoride
from .55 mg sodium fluoride
SUGAR FREE AND GLUTEN FREE
NO ARTIFICIAL COLORS OR DYES
DESCRIPTION SECTION
Description
Off-white to pale yellow, speckled, berry flavored, hexagon shaped chewable tablet. Debossed "173" on one side and scored on the other.
Quatrefolic Trademark and Patent property of Gnosis SpA.
SPL UNCLASSIFIED SECTION
Manufactured for:
BonGeo Pharmaceuticals, Inc.
Roseland, NJ 07068
Manufactured by:
Sancilio & Co., Inc.
Riviera Beach, FL 33404
WARNINGS SECTION
WARNING
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. IF OVERDOSAGE IS SUSPECTED, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY 1-800-222-1222. TABLET SHOULD BE CHEWED. THIS TABLET, AS WITH ALL TABLETS, IS NOT RECOMMENDED FOR CHILDREN UNDER AGE OF 4 DUE TO RISK OF CHOKING.
PRECAUTIONS SECTION
CAUTION
Do not use this product if you are allergic to any of the ingredients. Take this product at least 2 hours before or after taking any products containing calcium (including milk, yogurt, other dairy products) or aluminum/magnesium hydroxide (e.g., certain antacids/laxatives). Prolonged daily ingestion of excessive fluoride may result in varying degrees of dental fluorosis. Account for all daily sources of fluoride intake.
STORAGE AND HANDLING SECTION
OTHER INFORMATION
You may report serious side effects to 1-844-640-7607. Store in a cool, dry place at room temperature 20-25° Celsius (68-77° Fahrenheit) away from heat and sunlight. Store in original container.
The numeric identifier on this product's labeling is an assigned product code for use with pharmacy-level, health insurance and state level reimbursement programs and is not intended to denote registration with the FDA.
The manufacturer of this product requires that it be dispensed only under the order of a physician or licensed medical practitioner.
