Sanatos
Sanatos Nightime Softgel (HHH Pharma)
3dd3ed85-0c0a-8390-e063-6294a90a668e
HUMAN OTC DRUG LABEL
Sep 2, 2025
Pharmadel LLC
DUNS: 030129680
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
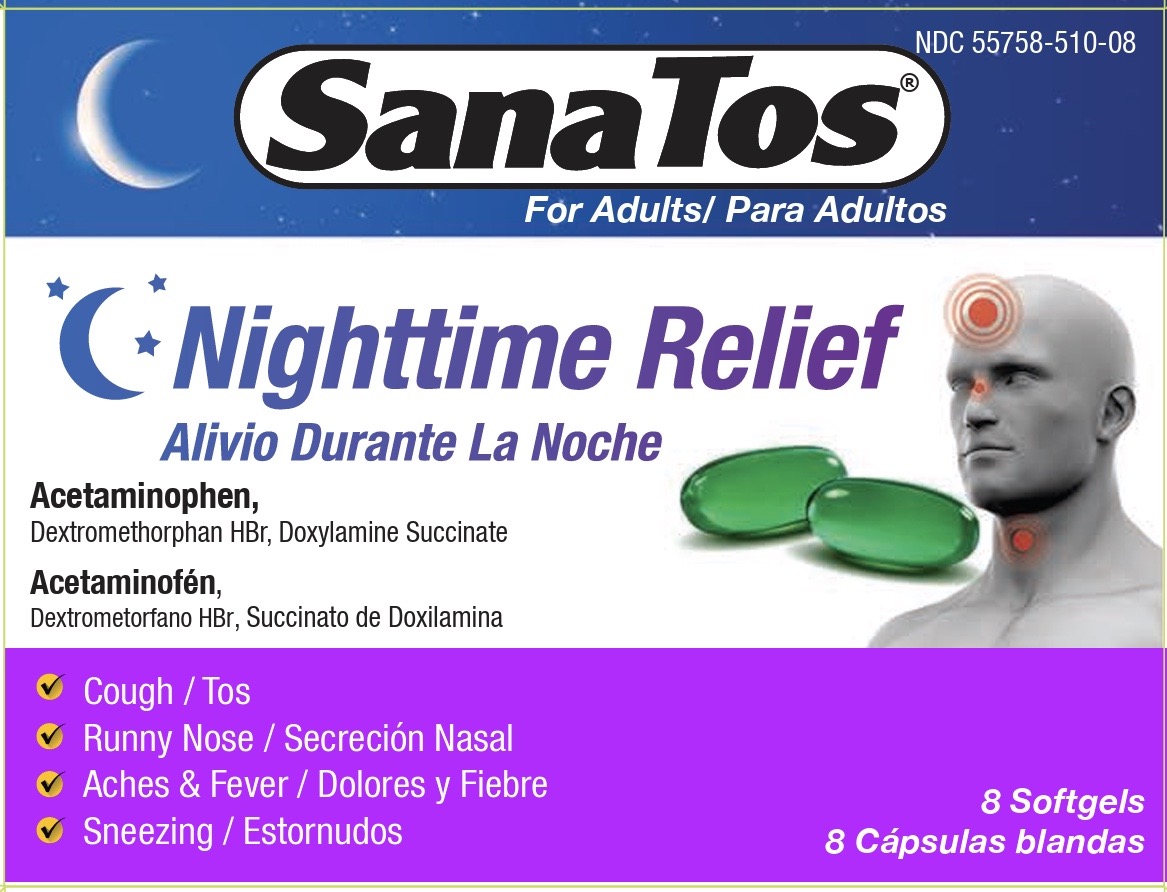
Acetaminophen, Dextromethorphan HBr, Doxylamine Succinate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
INDICATIONS & USAGE SECTION
Uses
Temporarily relieves cough-cold symptoms
- cough due to minor throat and bronchial irritation
- headache
- minor aches and pains
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes due to hay fever
- temporarily reduces fever
INACTIVE INGREDIENT SECTION
Inactive ingredients
D&C yellow #10, FD&C blue #1, gelatin, glycerin, polyethylene glycol 400, povidone k-30, propylene glycol, sorbitol sorbitan solution, water
OTC - QUESTIONS SECTION
Questions or comments?
+1-866-359-3478(M-F) 9 AM - 5 PM EST or www.pharmadel.com
OTHER SAFETY INFORMATION
Other information
- store between 68-77°F (20-25°C)
- avoid excessive heat
- do not use if blister pack is punctured or torn
SPL UNCLASSIFIED SECTION
Dist by/ por:
PHARMADEL LLC
New Castle, DE 19720
www.pharmadel.com
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients and Purposes
.
|
Active ingredients (in each softgel) |
Purposes |
|
Acetaminophen 325 mg.............. |
Pain reliever/ fever reducer |
|
Dextromethorphan HBr 15 mg ...... |
Cough suppressant |
|
Doxylamine succinate 6.25 mg ..... |
Antihistamine |
WARNINGS SECTION
Warnings
Liver warning:This product containsacetaminophen. Severe liver damage may occur if you take
- more than** 8 softgels in 24 hours,**which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks daily while using this product
Allergy alert: Acetaminophenmay cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- glaucoma
- a cough that is accompanied by excessive phlegm (mucus)
- a persistent or chronic cough as occurs with smoking, asthma or emphysema
- a breathing problem such as emphysema or chronic bronchitis
- difficulty urinating due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- may cause marked drowsiness
- excitability may occur, especially in children
- alcohol, sedatives and tranquilizers may increase drowsiness effect
- avoid alcoholic drinks when taking this product
- use caution when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- pain or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with a rash or a persistent headache that lasts.
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN.
In case of accidental overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
DOSAGE & ADMINISTRATION SECTION
Directions
|
Age |
Dose |
|
adults and children 12 years and older |
2 softgels every 6 hours,do not exceed 8 softgels in a 24 hour period |
|
children 4 to under 12 years |
consult a doctor |
|
children under 4 years |
do not use |
