Potassium Chloride in Lactated Ringers and Dextrose
INTRAVENOUS SOLUTIONS WITH POTASSIUM CHLORIDE
237b39fa-916e-4a54-99c8-4ae9ad54f056
HUMAN PRESCRIPTION DRUG LABEL
Jun 24, 2021
ICU Medical Inc.
DUNS: 118380146
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
POTASSIUM CHLORIDE, SODIUM CHLORIDE, CALCIUM CHLORIDE, SODIUM LACTATE, and DEXTROSE MONOHYDRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 1000 mL Bag Label
20 mEq POTASSIUM ADDED
1000 mL
NDC 0990-7111-09
20
mEq
POTASSIUM
CHLORIDE
in Lactated Ringer's
and 5% Dextrose
Injection, USP
EACH 100 mL CONTAINS POTASSIUM CHLORIDE
179 mg; SODIUM CHLORIDE 600 mg; SODIUM
LACTATE, ANHYDROUS 310 mg; CALCIUM CHLORIDE,
DIHYDRATE 20 mg; DEXTROSE, HYDROUS 5 g IN
WATER FOR INJECTION. MAY CONTAIN HCl FOR
pH ADJUSTMENT. ELECTROLYTES PER 1000 mL
(NOT INCLUDING IONS FOR pH ADJUSTMENT):
POTASSIUM 24 mEq; SODIUM 130 mEq; CHLORIDE
129 mEq; CALCIUM 2.7 mEq; LACTATE 28 mEq.
563 mOsmol/LITER (CALC.)
pH 4.9 (3.5 to 6.5)
NOT FOR USE IN THE TREATMENT OF LACTIC ACIDOSIS.
ADDITIVES MAY BE INCOMPATIBLE. CONSULT WITH
PHARMACIST, IF AVAILABLE. WHEN INTRODUCING
ADDITIVES, USE ASEPTIC TECHNIQUE, MIX
THOROUGHLY AND DO NOT STORE.
SINGLE-DOSE CONTAINER. FOR I.V. USE.
USUAL DOSAGE: SEE INSERT. STERILE, NONPYROGENIC.
USE ONLY IF SOLUTION IS CLEAR AND CONTAINER
IS UNDAMAGED. MUST NOT BE USED IN SERIES
CONNECTIONS.
Rx ONLY
3
V
CONTAINS DEHP
IMP0000048
ICU Medical, Inc.,
Lake Forest, Illinois, 60045, USA
icumedical

DESCRIPTION SECTION
DESCRIPTION
Intravenous solution with potassium chloride (I.V. solutions with KCl) is a sterile and nonpyrogenic solution in water for injection. This solution is for administration by intravenous infusion only.
See Table for summary of content and characteristics of this solution.
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded.
This solution is a parenteral fluid, nutrient and/or electrolyte replenisher.
Dextrose, USP is chemically designated D-glucose, monohydrate (C6H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:
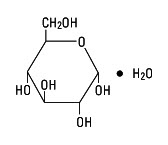
Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
Calcium Chloride, USP is chemically designated calcium chloride dihydrate (CaCl2 • 2H2O), white fragments or granules freely soluble in water.
Sodium Lactate, USP is chemically designated monosodium lactate [CH3CH(OH)COONa], a 60% aqueous solution miscible in water. It has the following structural formula:
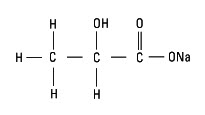
Water for Injection, USP is chemically designated H20.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
HOW SUPPLIED SECTION
HOW SUPPLIED
Intravenous solution with potassium chloride (I.V. solution with KCl) is supplied in single-dose flexible plastic container. See Table:
Potassium Chloride
in Lactated Ringer’s
and 5% Dextrose
Injection, USP
|
List No. |
mEq Potassium Added |
Size (mL) |
COMPOSITION (g/L) |
Calculated Osmolarity (mOsmol/L) | ||||
|
Dextrose, Hydrous |
Potassium Chloride |
Sodium Chloride |
Sodium Lactate, Anhydrous |
Calcium Chloride Dihydrate | ||||
|
7111 |
20 mEq |
1000 |
50 |
1.79 |
6 |
3.1 |
0.2 |
563 |
|
NDC No. | ||||||||
|
0409-7111-09 | ||||||||
|
0990-7111-09 |
Potassium Chloride
in Lactated Ringer’s
and 5% Dextrose
Injection, USP (Continued)
|
pH |
Approx. Ionic Concentrations (mEq/L) |
Approx. kcal/L | ||||
|
Calcium (Ca++) |
Sodium (Na**+****)** |
Potassium (K**+****)** |
Chloride (Cl¯) |
Lactate | ||
|
4.9 (3.5 to 6.5) |
2.7 |
130 |
24 |
129 |
28 |
179 |
ICU Medical is transitioning NDC codes from the "0409" to a "0990" labeler code. Both NDC codes are expected to be in the market for a period of time.
May contain HCl for pH adjustment.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Revised: March, 2020
IFU0000167
ICU Medical, Inc., Lake Forest, Illinois, 60045, USA
