Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
VUSION
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – 0.25%, 15% and 81.35%
NDC 0378-8222-50
Miconazole
Nitrate,
0.25%
Zinc Oxide,
15%
and White
Petrolatum,
81.35% Ointment
For Topical Use Only
Usual Dosage: See package insert.
Caution: Not for oral, ophthalmic, or
intravaginal use. Keep out of reach of
children. If seal is damaged or
punctured, do not use, and return
product to place of purchase.
Description: Each gram contains 2.5 mg
miconazole nitrate USP, 150 mg zinc
oxide USP, and 813.5 mg white
petrolatum USP containing butylated
hydroxytoluene, trihyforxystearin, and
Chemoderm 1001/B fragrance.
Store at 20º to 25ºC (68º to 77ºF).
[See USP Controlled Room Temperature.]
See flap for lot number and expiration date.
For additional information, call Mylan at
1-877-446-3679 (1-877-4-INFO-RX).
Serious side effects associated with the
use of this product may be reported to
this number.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in Canada
CON:8222:50:1C:R1 301922-01
Mylan.com

DESCRIPTION SECTION
11 DESCRIPTION
Miconazole nitrate, zinc oxide and white petrolatum ointment contains the synthetic antifungal agent, miconazole nitrate (0.25%) USP, zinc oxide (15%) USP, and white petrolatum (81.35%) USP.
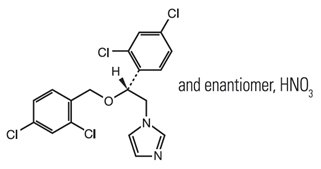
The chemical name of miconazole nitrate is 1-[2, 4-dichloro-ß-{(2,4-dichlorobenzyl)oxy} phenethyl] imidazole mononitrate with empirical formula C18H14Cl4N2O•HNO3 and molecular weight of 479.15. The structural formula of miconazole nitrate is as follows:
The zinc oxide has an empirical formula of ZnO and a molecular weight of 81.39.
The white petrolatum, which is obtained from petroleum and is wholly or nearly decolorized, is a purified mixture of semisolid saturated hydrocarbons having the general chemical formula CnH2n+2. The hydrocarbons consist mainly of branched and unbranched chains. White petrolatum contains butylated hydroxytoluene (BHT) as stabilizer.
Each gram of miconazole nitrate, zinc oxide and white petrolatum ointment contains 2.5 mg of miconazole nitrate, USP, 150 mg of zinc oxide, USP and 813.5 mg of white petrolatum, USP containing butylated hydroxytoluene, trihydroxystearin, and Chemoderm 1001/B fragrance.
Miconazole nitrate, zinc oxide and white petrolatum ointment is a smooth, uniform, white ointment.
