Tripleantibioticointmentandpainrelief
Triple Antibiotic Ointment + Pain Relief
34f1db74-6506-d53d-e063-6394a90ac400
HUMAN OTC DRUG LABEL
May 12, 2025
Trifecta Pharmaceuticals USA
DUNS: 079424163
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Bacitracin,Neomycin,Polymxin, Pramoxine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
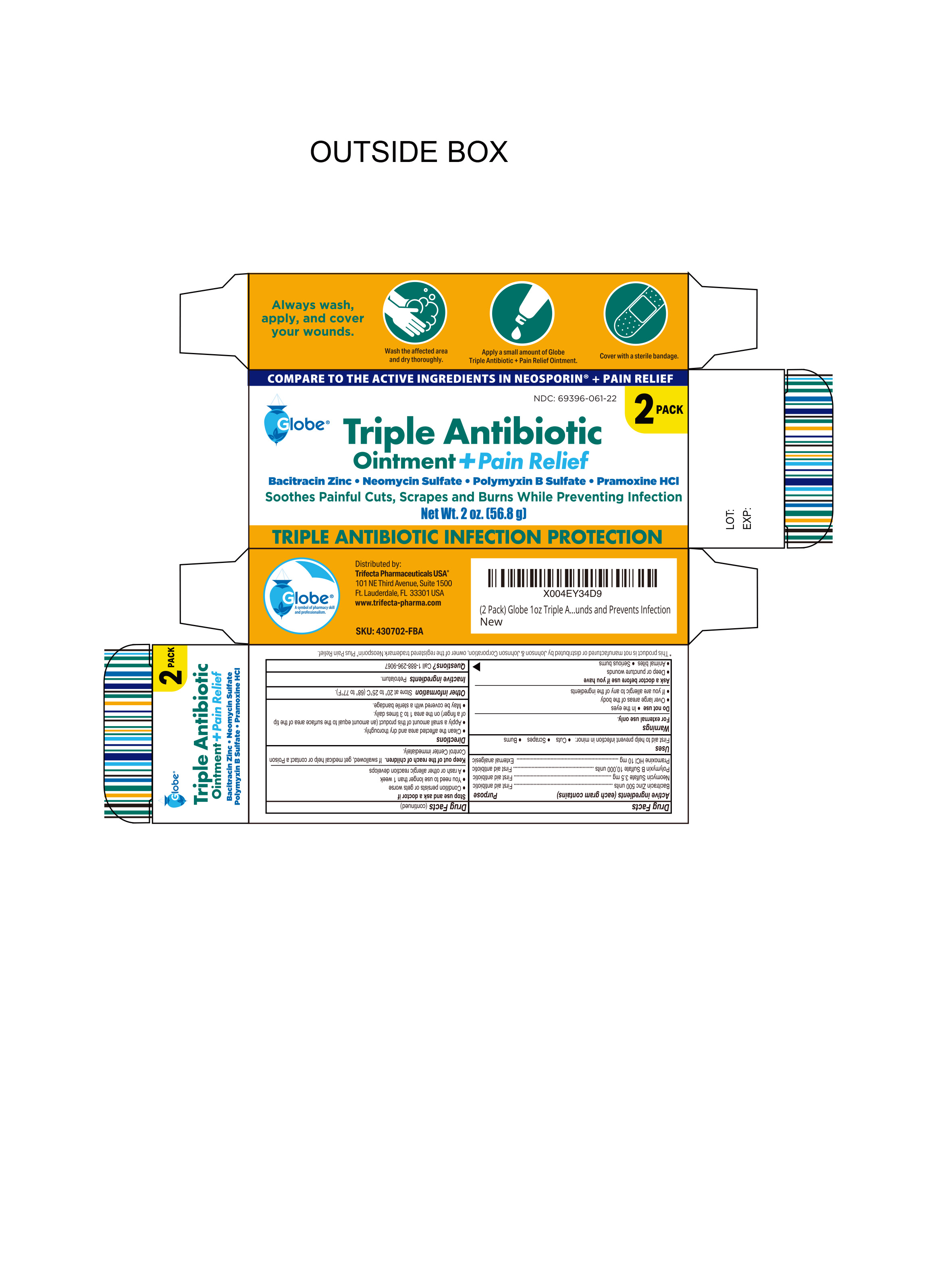
INDICATIONS & USAGE SECTION
Uses
First Aid to help prevent infection in minor:
- Cuts
- Scrapes
- Burns
OTC - ASK DOCTOR SECTION
Ask a Doctor before Use
Ask Doctor before use if you have:
- Deep or puncture wouns
- Animal bites
- Serious burns
SPL UNCLASSIFIED SECTION
Distributed By:
Trifecta Pharmaceuticals USA ®
101 NE Third Avenue, Suite 1500
Fort Lauderdale, FL. 33301 USA
www.trifecta-pharma.com
SKU: 8801304-FBA
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Pramoxine HCL 10mg
OTC - PURPOSE SECTION
Purpose
External Analgesic
WARNINGS SECTION
Warnings
For external use only. Do not use:
- In eyes
- Over large areas of the body
- If you are allergic to any of the ingredients
OTC - STOP USE SECTION
Stop Use and ask a Doctor if:
- Condition persists or gets worse
- You need to use longer than 1 week
- A rash or other allergic reaction develops
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of Reach of Children
If Swallowed, get medical help or contact a Poison Control Center right away
DOSAGE & ADMINISTRATION SECTION
Directions
- Clean the affected area thoroughly
- Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- May be covered with a sterile bandage
STORAGE AND HANDLING SECTION
Other Information
- To Open: Unscrew cap, pull tab to remove foil seal
- Store at 20° to 25°C (68° to 77°F)
- See carton or tube crimp for lot number and expiration date
INACTIVE INGREDIENT SECTION
Inactive Ingredient:
Petrolatum
OTC - QUESTIONS SECTION
Questions?
Call 1-888-296-9067
